Chapter 5 Manipulating and analyzing data with dplyr
Learning Objectives
Describe the purpose of the
dplyrandtidyrpackages.Describe several of their functions that are extremely useful to manipulate data.
Describe the concept of a wide and a long table format, and see how to reshape a data frame from one format to the other one.
5.1 Data Manipulation using dplyr and tidyr
Bracket subsetting is handy, but it can be cumbersome and difficult to read, especially for complicated operations.
Some packages can greatly facilitate our task when we manipulate data.
Packages in R are basically sets of additional functions that let you
do more stuff. The functions we’ve been using so far, like str() or
data.frame(), come built into R; Loading packages can give you access to other
specific functions. Before you use a package for the first time you need to install
it on your machine, and then you should import it in every subsequent
R session when you need it.
The package
dplyrprovides powerful tools for data manipulation tasks. It is built to work directly with data frames, with many manipulation tasks optimized.As we will see latter on, sometimes we want a data frame to be reshaped to be able to do some specific analyses or for visualization. The package
tidyraddresses this common problem of reshaping data and provides tools for manipulating data in a tidy way.
To learn more about dplyr and tidyr after the workshop,
you may want to check out this handy data transformation with
dplyr
cheatsheet
and this one about
tidyr.
- The
tidyversepackage is an “umbrella-package” that installs several useful packages for data analysis which work well together, such astidyr,dplyr,ggplot2,tibble, etc. These packages help us to work and interact with the data. They allow us to do many things with your data, such as subsetting, transforming, visualizing, etc.
To install and load the tidyverse package type:
BiocManager::install("tidyverse")## load the tidyverse packages, incl. dplyr
library("tidyverse")5.2 Loading data with tidyverse
Instead of read.csv(), we will read in our data using the read_csv() function, from the
tidyverse package readr, .
rna <- read_csv("data/rnaseq.csv")
## view the data
rna## # A tibble: 32,428 × 19
## gene sample expression organism age sex infection strain time tissue
## <chr> <chr> <dbl> <chr> <dbl> <chr> <chr> <chr> <dbl> <chr>
## 1 Asl GSM254… 1170 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 2 Apod GSM254… 36194 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 3 Cyp2d22 GSM254… 4060 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 4 Klk6 GSM254… 287 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 5 Fcrls GSM254… 85 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 6 Slc2a4 GSM254… 782 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 7 Exd2 GSM254… 1619 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 8 Gjc2 GSM254… 288 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 9 Plp1 GSM254… 43217 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 10 Gnb4 GSM254… 1071 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## # … with 32,418 more rows, and 9 more variables: mouse <dbl>, ENTREZID <dbl>,
## # product <chr>, ensembl_gene_id <chr>, external_synonym <chr>,
## # chromosome_name <chr>, gene_biotype <chr>, phenotype_description <chr>,
## # hsapiens_homolog_associated_gene_name <chr>Notice that the class of the data is now referred to as a “tibble.”
Tibbles tweak some of the behaviors of the data frame objects we introduced in the previously. The data structure is very similar to a data frame. For our purposes the only differences are that:
It displays the data type of each column under its name. Note that <
dbl> is a data type defined to hold numeric values with decimal points.It only prints the first few rows of data and only as many columns as fit on one screen.
We are now going to learn some of the most common dplyr functions:
-
select(): subset columns -
filter(): subset rows on conditions -
mutate(): create new columns by using information from other columns -
group_by()andsummarize(): create summary statistics on grouped data -
arrange(): sort results -
count(): count discrete values
5.3 Selecting columns and filtering rows
To select columns of a data frame, use select(). The first argument
to this function is the data frame (rna), and the subsequent
arguments are the columns to keep.
select(rna, gene, sample, tissue, expression)## # A tibble: 32,428 × 4
## gene sample tissue expression
## <chr> <chr> <chr> <dbl>
## 1 Asl GSM2545336 Cerebellum 1170
## 2 Apod GSM2545336 Cerebellum 36194
## 3 Cyp2d22 GSM2545336 Cerebellum 4060
## 4 Klk6 GSM2545336 Cerebellum 287
## 5 Fcrls GSM2545336 Cerebellum 85
## 6 Slc2a4 GSM2545336 Cerebellum 782
## 7 Exd2 GSM2545336 Cerebellum 1619
## 8 Gjc2 GSM2545336 Cerebellum 288
## 9 Plp1 GSM2545336 Cerebellum 43217
## 10 Gnb4 GSM2545336 Cerebellum 1071
## # … with 32,418 more rowsTo select all columns except certain ones, put a “-” in front of the variable to exclude it.
select(rna, -tissue, -organism)## # A tibble: 32,428 × 17
## gene sample expression age sex infection strain time mouse ENTREZID
## <chr> <chr> <dbl> <dbl> <chr> <chr> <chr> <dbl> <dbl> <dbl>
## 1 Asl GSM2545336 1170 8 Female Influenz… C57BL… 8 14 109900
## 2 Apod GSM2545336 36194 8 Female Influenz… C57BL… 8 14 11815
## 3 Cyp2d22 GSM2545336 4060 8 Female Influenz… C57BL… 8 14 56448
## 4 Klk6 GSM2545336 287 8 Female Influenz… C57BL… 8 14 19144
## 5 Fcrls GSM2545336 85 8 Female Influenz… C57BL… 8 14 80891
## 6 Slc2a4 GSM2545336 782 8 Female Influenz… C57BL… 8 14 20528
## 7 Exd2 GSM2545336 1619 8 Female Influenz… C57BL… 8 14 97827
## 8 Gjc2 GSM2545336 288 8 Female Influenz… C57BL… 8 14 118454
## 9 Plp1 GSM2545336 43217 8 Female Influenz… C57BL… 8 14 18823
## 10 Gnb4 GSM2545336 1071 8 Female Influenz… C57BL… 8 14 14696
## # … with 32,418 more rows, and 7 more variables: product <chr>,
## # ensembl_gene_id <chr>, external_synonym <chr>, chromosome_name <chr>,
## # gene_biotype <chr>, phenotype_description <chr>,
## # hsapiens_homolog_associated_gene_name <chr>This will select all the variables in rna except tissue
and organism.
To choose rows based on a specific criteria, use filter():
filter(rna, sex == "Male")## # A tibble: 14,740 × 19
## gene sample expression organism age sex infection strain time tissue
## <chr> <chr> <dbl> <chr> <dbl> <chr> <chr> <chr> <dbl> <chr>
## 1 Asl GSM254… 626 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## 2 Apod GSM254… 13021 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## 3 Cyp2d22 GSM254… 2171 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## 4 Klk6 GSM254… 448 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## 5 Fcrls GSM254… 180 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## 6 Slc2a4 GSM254… 313 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## 7 Exd2 GSM254… 2366 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## 8 Gjc2 GSM254… 310 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## 9 Plp1 GSM254… 53126 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## 10 Gnb4 GSM254… 1355 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## # … with 14,730 more rows, and 9 more variables: mouse <dbl>, ENTREZID <dbl>,
## # product <chr>, ensembl_gene_id <chr>, external_synonym <chr>,
## # chromosome_name <chr>, gene_biotype <chr>, phenotype_description <chr>,
## # hsapiens_homolog_associated_gene_name <chr>filter(rna, sex == "Male" & infection == "NonInfected")## # A tibble: 4,422 × 19
## gene sample expression organism age sex infection strain time tissue
## <chr> <chr> <dbl> <chr> <dbl> <chr> <chr> <chr> <dbl> <chr>
## 1 Asl GSM254… 535 Mus mus… 8 Male NonInfec… C57BL… 0 Cereb…
## 2 Apod GSM254… 13668 Mus mus… 8 Male NonInfec… C57BL… 0 Cereb…
## 3 Cyp2d22 GSM254… 2008 Mus mus… 8 Male NonInfec… C57BL… 0 Cereb…
## 4 Klk6 GSM254… 1101 Mus mus… 8 Male NonInfec… C57BL… 0 Cereb…
## 5 Fcrls GSM254… 375 Mus mus… 8 Male NonInfec… C57BL… 0 Cereb…
## 6 Slc2a4 GSM254… 249 Mus mus… 8 Male NonInfec… C57BL… 0 Cereb…
## 7 Exd2 GSM254… 3126 Mus mus… 8 Male NonInfec… C57BL… 0 Cereb…
## 8 Gjc2 GSM254… 791 Mus mus… 8 Male NonInfec… C57BL… 0 Cereb…
## 9 Plp1 GSM254… 98658 Mus mus… 8 Male NonInfec… C57BL… 0 Cereb…
## 10 Gnb4 GSM254… 2437 Mus mus… 8 Male NonInfec… C57BL… 0 Cereb…
## # … with 4,412 more rows, and 9 more variables: mouse <dbl>, ENTREZID <dbl>,
## # product <chr>, ensembl_gene_id <chr>, external_synonym <chr>,
## # chromosome_name <chr>, gene_biotype <chr>, phenotype_description <chr>,
## # hsapiens_homolog_associated_gene_name <chr>Now let’s imagine we are interested in the human homologs of the mouse
genes analysed in this dataset. This information can be found in the
last column of the rna tibble, named hsapiens_homolog_associated_gene_name.
Some mouse gene have no human homologs. These can be retrieved using a filter()
in the chain, and the is.na() function that determines whether something is an NA.
rna_NA <- filter(rna, is.na(hsapiens_homolog_associated_gene_name))
select(rna_NA, gene, hsapiens_homolog_associated_gene_name)## # A tibble: 4,774 × 2
## gene hsapiens_homolog_associated_gene_name
## <chr> <chr>
## 1 Prodh <NA>
## 2 Icosl <NA>
## 3 Tssk5 <NA>
## 4 Vmn2r1 <NA>
## 5 Gm10654 <NA>
## 6 Hexa <NA>
## 7 Sult1a1 <NA>
## 8 Gm6277 <NA>
## 9 Amt <NA>
## 10 Tmem198b <NA>
## # … with 4,764 more rowsIf we want to keep only mouse gene that have a human homolog, we can insert a “!”
symbol that negates the result, so we’re asking for every row where
hsapiens_homolog_associated_gene_name is not an NA.
rna_no_NA <- filter(rna, !is.na(hsapiens_homolog_associated_gene_name))
select(rna_no_NA, gene, hsapiens_homolog_associated_gene_name)## # A tibble: 27,654 × 2
## gene hsapiens_homolog_associated_gene_name
## <chr> <chr>
## 1 Asl ASL
## 2 Apod APOD
## 3 Cyp2d22 CYP2D6
## 4 Klk6 KLK6
## 5 Fcrls FCRL4
## 6 Slc2a4 SLC2A4
## 7 Exd2 EXD2
## 8 Gjc2 GJC2
## 9 Plp1 PLP1
## 10 Gnb4 GNB4
## # … with 27,644 more rows5.4 Pipes
What if you want to select and filter at the same time? There are three ways to do this: use intermediate steps, nested functions, or pipes.
With intermediate steps, you create a temporary data frame and use that as input to the next function, like this:
rna2 <- filter(rna, sex == "Male")
rna3 <- select(rna2, gene, sample, tissue, expression)
rna3## # A tibble: 14,740 × 4
## gene sample tissue expression
## <chr> <chr> <chr> <dbl>
## 1 Asl GSM2545340 Cerebellum 626
## 2 Apod GSM2545340 Cerebellum 13021
## 3 Cyp2d22 GSM2545340 Cerebellum 2171
## 4 Klk6 GSM2545340 Cerebellum 448
## 5 Fcrls GSM2545340 Cerebellum 180
## 6 Slc2a4 GSM2545340 Cerebellum 313
## 7 Exd2 GSM2545340 Cerebellum 2366
## 8 Gjc2 GSM2545340 Cerebellum 310
## 9 Plp1 GSM2545340 Cerebellum 53126
## 10 Gnb4 GSM2545340 Cerebellum 1355
## # … with 14,730 more rowsThis is readable, but can clutter up your workspace with lots of intermediate objects that you have to name individually. With multiple steps, that can be hard to keep track of.
You can also nest functions (i.e. one function inside of another), like this:
rna3 <- select(filter(rna, sex == "Male"), gene, sample, tissue, expression)
rna3## # A tibble: 14,740 × 4
## gene sample tissue expression
## <chr> <chr> <chr> <dbl>
## 1 Asl GSM2545340 Cerebellum 626
## 2 Apod GSM2545340 Cerebellum 13021
## 3 Cyp2d22 GSM2545340 Cerebellum 2171
## 4 Klk6 GSM2545340 Cerebellum 448
## 5 Fcrls GSM2545340 Cerebellum 180
## 6 Slc2a4 GSM2545340 Cerebellum 313
## 7 Exd2 GSM2545340 Cerebellum 2366
## 8 Gjc2 GSM2545340 Cerebellum 310
## 9 Plp1 GSM2545340 Cerebellum 53126
## 10 Gnb4 GSM2545340 Cerebellum 1355
## # … with 14,730 more rowsThis is handy, but can be difficult to read if too many functions are nested, as R evaluates the expression from the inside out (in this case, filtering, then selecting).
The last option, pipes, are a recent addition to R. Pipes let you take the output of one function and send it directly to the next, which is useful when you need to do many things to the same dataset.
Pipes in R look like %>% and are made available via the magrittr package,
installed automatically with dplyr. If you use RStudio, you can type the pipe with Ctrl
+ Shift + M if you have a PC or Cmd +
Shift + M if you have a Mac.
In the above code, we use the pipe to send the rna dataset first through
filter() to keep rows where sex is Male, then through select()
to keep only the gene, sample, tissue, and expressioncolumns.
The pipe %>% takes the object on its left and passes it directly as the first
argument to the function on its right, we don’t need to explicitly include the data frame
as an argument to the filter() and select() functions any more.
rna %>%
filter(sex == "Male") %>%
select(gene, sample, tissue, expression)## # A tibble: 14,740 × 4
## gene sample tissue expression
## <chr> <chr> <chr> <dbl>
## 1 Asl GSM2545340 Cerebellum 626
## 2 Apod GSM2545340 Cerebellum 13021
## 3 Cyp2d22 GSM2545340 Cerebellum 2171
## 4 Klk6 GSM2545340 Cerebellum 448
## 5 Fcrls GSM2545340 Cerebellum 180
## 6 Slc2a4 GSM2545340 Cerebellum 313
## 7 Exd2 GSM2545340 Cerebellum 2366
## 8 Gjc2 GSM2545340 Cerebellum 310
## 9 Plp1 GSM2545340 Cerebellum 53126
## 10 Gnb4 GSM2545340 Cerebellum 1355
## # … with 14,730 more rowsSome may find it helpful to read the pipe like the word “then.” For instance,
in the above example, we took the data frame rna, then we filtered
for rows with sex == "Male", then we selected columns gene, sample,
tissue, and expression.
The dplyr functions by themselves are somewhat
simple, but by combining them into linear workflows with the pipe, we can accomplish
more complex manipulations of data frames.
If we want to create a new object with this smaller version of the data, we can assign it a new name:
rna3 <- rna %>%
filter(sex == "Male") %>%
select(gene, sample, tissue, expression)
rna3## # A tibble: 14,740 × 4
## gene sample tissue expression
## <chr> <chr> <chr> <dbl>
## 1 Asl GSM2545340 Cerebellum 626
## 2 Apod GSM2545340 Cerebellum 13021
## 3 Cyp2d22 GSM2545340 Cerebellum 2171
## 4 Klk6 GSM2545340 Cerebellum 448
## 5 Fcrls GSM2545340 Cerebellum 180
## 6 Slc2a4 GSM2545340 Cerebellum 313
## 7 Exd2 GSM2545340 Cerebellum 2366
## 8 Gjc2 GSM2545340 Cerebellum 310
## 9 Plp1 GSM2545340 Cerebellum 53126
## 10 Gnb4 GSM2545340 Cerebellum 1355
## # … with 14,730 more rows► Question
Using pipes, subset the rna data to keep genes with an expression higher
than 50000 in female mice at time 0, and retain only the columns gene,
sample, time, expression and age
► Solution
5.5 Mutate
Frequently you’ll want to create new columns based on the values of existing
columns, for example to do unit conversions, or to find the ratio of values in two
columns. For this we’ll use mutate().
To create a new column of time in hours:
rna %>%
mutate(time_hours = time * 24) %>%
select(time, time_hours) ## # A tibble: 32,428 × 2
## time time_hours
## <dbl> <dbl>
## 1 8 192
## 2 8 192
## 3 8 192
## 4 8 192
## 5 8 192
## 6 8 192
## 7 8 192
## 8 8 192
## 9 8 192
## 10 8 192
## # … with 32,418 more rowsYou can also create a second new column based on the first new column within the same call of mutate():
rna %>%
mutate(time_hours = time * 24,
time_mn = time_hours * 60) %>%
select(time, time_hours, time_mn) ## # A tibble: 32,428 × 3
## time time_hours time_mn
## <dbl> <dbl> <dbl>
## 1 8 192 11520
## 2 8 192 11520
## 3 8 192 11520
## 4 8 192 11520
## 5 8 192 11520
## 6 8 192 11520
## 7 8 192 11520
## 8 8 192 11520
## 9 8 192 11520
## 10 8 192 11520
## # … with 32,418 more rows► Question
Create a new data frame from the rna data that meets the following criteria:
contains only the gene, chromosome_name, phenotype_description, sample,
and expression columns and a new column giving the log expression the gene.
This data frame must only contain genes located on autosomes and associated with a phenotype_description.
Hint: think about how the commands should be ordered to produce this data frame!
► Solution
5.6 Split-apply-combine data analysis
Many data analysis tasks can be approached using the
split-apply-combine paradigm: split the data into groups, apply some
analysis to each group, and then combine the results. dplyr
makes this very easy through the use of the group_by() function.
rna %>%
group_by(gene)## # A tibble: 32,428 × 19
## # Groups: gene [1,474]
## gene sample expression organism age sex infection strain time tissue
## <chr> <chr> <dbl> <chr> <dbl> <chr> <chr> <chr> <dbl> <chr>
## 1 Asl GSM254… 1170 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 2 Apod GSM254… 36194 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 3 Cyp2d22 GSM254… 4060 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 4 Klk6 GSM254… 287 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 5 Fcrls GSM254… 85 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 6 Slc2a4 GSM254… 782 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 7 Exd2 GSM254… 1619 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 8 Gjc2 GSM254… 288 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 9 Plp1 GSM254… 43217 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 10 Gnb4 GSM254… 1071 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## # … with 32,418 more rows, and 9 more variables: mouse <dbl>, ENTREZID <dbl>,
## # product <chr>, ensembl_gene_id <chr>, external_synonym <chr>,
## # chromosome_name <chr>, gene_biotype <chr>, phenotype_description <chr>,
## # hsapiens_homolog_associated_gene_name <chr>The group_by() function doesn’t perform any data processing, it
groups the data into subsets: in the example above, our initial
tibble of 32428 observations is split into
1474 groups based on the gene variable.
We could similarly decide to group the tibble by the samples:
rna %>%
group_by(sample)## # A tibble: 32,428 × 19
## # Groups: sample [22]
## gene sample expression organism age sex infection strain time tissue
## <chr> <chr> <dbl> <chr> <dbl> <chr> <chr> <chr> <dbl> <chr>
## 1 Asl GSM254… 1170 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 2 Apod GSM254… 36194 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 3 Cyp2d22 GSM254… 4060 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 4 Klk6 GSM254… 287 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 5 Fcrls GSM254… 85 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 6 Slc2a4 GSM254… 782 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 7 Exd2 GSM254… 1619 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 8 Gjc2 GSM254… 288 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 9 Plp1 GSM254… 43217 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 10 Gnb4 GSM254… 1071 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## # … with 32,418 more rows, and 9 more variables: mouse <dbl>, ENTREZID <dbl>,
## # product <chr>, ensembl_gene_id <chr>, external_synonym <chr>,
## # chromosome_name <chr>, gene_biotype <chr>, phenotype_description <chr>,
## # hsapiens_homolog_associated_gene_name <chr>Here our initial tibble of 32428 observations is split into
22 groups based on the sample variable.
Once the data have been combined, subsequent operations will be applied on each group independently.
5.6.1 The summarize() function
group_by() is often used together with summarize(), which
collapses each group into a single-row summary of that group.
group_by() takes as arguments the column names that contain the
categorical variables for which you want to calculate the summary
statistics. So to compute the mean expression by gene:
rna %>%
group_by(gene) %>%
summarize(mean_expression = mean(expression))## # A tibble: 1,474 × 2
## gene mean_expression
## <chr> <dbl>
## 1 AI504432 1053.
## 2 AW046200 131.
## 3 AW551984 295.
## 4 Aamp 4751.
## 5 Abca12 4.55
## 6 Abcc8 2498.
## 7 Abhd14a 525.
## 8 Abi2 4909.
## 9 Abi3bp 1002.
## 10 Abl2 2124.
## # … with 1,464 more rowsWe could also want to calculate the mean expression levels of all genes in each sample:
rna %>%
group_by(sample) %>%
summarize(mean_expression = mean(expression))## # A tibble: 22 × 2
## sample mean_expression
## <chr> <dbl>
## 1 GSM2545336 2064.
## 2 GSM2545337 1766.
## 3 GSM2545338 1668.
## 4 GSM2545339 1697.
## 5 GSM2545340 1682.
## 6 GSM2545341 1638.
## 7 GSM2545342 1595.
## 8 GSM2545343 2108.
## 9 GSM2545344 1714.
## 10 GSM2545345 1701.
## # … with 12 more rowsBut we can can also group by multiple columns:
rna %>%
group_by(gene, infection, time) %>%
summarize(mean_expression = mean(expression))## `summarise()` has grouped output by 'gene', 'infection'. You can override using the `.groups` argument.## # A tibble: 4,422 × 4
## # Groups: gene, infection [2,948]
## gene infection time mean_expression
## <chr> <chr> <dbl> <dbl>
## 1 AI504432 InfluenzaA 4 1104.
## 2 AI504432 InfluenzaA 8 1014
## 3 AI504432 NonInfected 0 1034.
## 4 AW046200 InfluenzaA 4 152.
## 5 AW046200 InfluenzaA 8 81
## 6 AW046200 NonInfected 0 155.
## 7 AW551984 InfluenzaA 4 302.
## 8 AW551984 InfluenzaA 8 342.
## 9 AW551984 NonInfected 0 238
## 10 Aamp InfluenzaA 4 4870
## # … with 4,412 more rowsOnce the data is grouped, you can also summarize multiple variables at the same
time (and not necessarily on the same variable). For instance, we could add a
column indicating the median expression by gene and by condition:
rna %>%
group_by(gene, infection, time) %>%
summarize(mean_expression = mean(expression),
median_expression = median(expression)) ## `summarise()` has grouped output by 'gene', 'infection'. You can override using the `.groups` argument.## # A tibble: 4,422 × 5
## # Groups: gene, infection [2,948]
## gene infection time mean_expression median_expression
## <chr> <chr> <dbl> <dbl> <dbl>
## 1 AI504432 InfluenzaA 4 1104. 1094.
## 2 AI504432 InfluenzaA 8 1014 985
## 3 AI504432 NonInfected 0 1034. 1016
## 4 AW046200 InfluenzaA 4 152. 144.
## 5 AW046200 InfluenzaA 8 81 82
## 6 AW046200 NonInfected 0 155. 163
## 7 AW551984 InfluenzaA 4 302. 245
## 8 AW551984 InfluenzaA 8 342. 287
## 9 AW551984 NonInfected 0 238 265
## 10 Aamp InfluenzaA 4 4870 4708
## # … with 4,412 more rows► Question
Calculate the mean expression level of gene “Dok3” by timepoints.
► Solution
5.6.2 Counting
When working with data, we often want to know the number of observations found
for each factor or combination of factors. For this task, dplyr provides
count(). For example, if we wanted to count the number of rows of data for
each infected and non infected samples, we would do:
rna %>%
count(infection)## # A tibble: 2 × 2
## infection n
## <chr> <int>
## 1 InfluenzaA 22110
## 2 NonInfected 10318The count() function is shorthand for something we’ve already seen: grouping by a variable, and summarizing it by counting the number of observations in that group. In other words, rna %>% count() is equivalent to:
rna %>%
group_by(infection) %>%
summarise(n = n())## # A tibble: 2 × 2
## infection n
## <chr> <int>
## 1 InfluenzaA 22110
## 2 NonInfected 10318Previous example shows the use of count() to count the number of rows/observations
for one factor (i.e., infection).
If we wanted to count combination of factors, such as infection and time,
we would specify the first and the second factor as the arguments of count():
rna %>%
count(infection, time)## # A tibble: 3 × 3
## infection time n
## <chr> <dbl> <int>
## 1 InfluenzaA 4 11792
## 2 InfluenzaA 8 10318
## 3 NonInfected 0 10318which is equivalent to this:
rna %>%
group_by(infection, time) %>%
summarize(n = n())## `summarise()` has grouped output by 'infection'. You can override using the `.groups` argument.## # A tibble: 3 × 3
## # Groups: infection [2]
## infection time n
## <chr> <dbl> <int>
## 1 InfluenzaA 4 11792
## 2 InfluenzaA 8 10318
## 3 NonInfected 0 10318It is sometimes useful to sort the result to facilitate the comparisons.
We can use arrange() to sort the table.
For instance, we might want to arrange the table above by time:
rna %>%
count(infection, time) %>%
arrange(time)## # A tibble: 3 × 3
## infection time n
## <chr> <dbl> <int>
## 1 NonInfected 0 10318
## 2 InfluenzaA 4 11792
## 3 InfluenzaA 8 10318or by counts:
rna %>%
count(infection, time) %>%
arrange(n)## # A tibble: 3 × 3
## infection time n
## <chr> <dbl> <int>
## 1 InfluenzaA 8 10318
## 2 NonInfected 0 10318
## 3 InfluenzaA 4 11792To sort in descending order, we need to add the desc() function:
rna %>%
count(infection, time) %>%
arrange(desc(n))## # A tibble: 3 × 3
## infection time n
## <chr> <dbl> <int>
## 1 InfluenzaA 4 11792
## 2 InfluenzaA 8 10318
## 3 NonInfected 0 10318► Question
- How many genes were analysed in each sample?
► Solution
► Question
- Use
group_by()andsummarize()to evaluate the sequencing depth (the sum of all counts) in each sample. Which sample has the highest sequencing depth?
► Solution
► Question
- Pick one sample and evaluate the number of genes by biotype
► Solution
► Question
- Identify genes associated with “abnormal DNA methylation” phenotype description, and calculate their mean expression (in log) at time 0, time 4 and time 8.
► Solution
5.7 Reshaping data
In the rna tibble, the rows contain expression values (the unit) that are
associated with a combination of 2 other variables: gene and sample.
All the other columns correspond to variables describing either the sample (organism, age, sex,…) or the gene (gene_biotype, ENTREZ_ID, product…). The variables that don’t change with genes or with samples will have the same value in all the rows.
rna %>%
arrange(gene) ## # A tibble: 32,428 × 19
## gene sample expression organism age sex infection strain time tissue
## <chr> <chr> <dbl> <chr> <dbl> <chr> <chr> <chr> <dbl> <chr>
## 1 AI504432 GSM25… 1230 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 2 AI504432 GSM25… 1085 Mus mus… 8 Fema… NonInfec… C57BL… 0 Cereb…
## 3 AI504432 GSM25… 969 Mus mus… 8 Fema… NonInfec… C57BL… 0 Cereb…
## 4 AI504432 GSM25… 1284 Mus mus… 8 Fema… Influenz… C57BL… 4 Cereb…
## 5 AI504432 GSM25… 966 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## 6 AI504432 GSM25… 918 Mus mus… 8 Male Influenz… C57BL… 8 Cereb…
## 7 AI504432 GSM25… 985 Mus mus… 8 Fema… Influenz… C57BL… 8 Cereb…
## 8 AI504432 GSM25… 972 Mus mus… 8 Male NonInfec… C57BL… 0 Cereb…
## 9 AI504432 GSM25… 1000 Mus mus… 8 Fema… Influenz… C57BL… 4 Cereb…
## 10 AI504432 GSM25… 816 Mus mus… 8 Male Influenz… C57BL… 4 Cereb…
## # … with 32,418 more rows, and 9 more variables: mouse <dbl>, ENTREZID <dbl>,
## # product <chr>, ensembl_gene_id <chr>, external_synonym <chr>,
## # chromosome_name <chr>, gene_biotype <chr>, phenotype_description <chr>,
## # hsapiens_homolog_associated_gene_name <chr>This structure is called a long-format, as one column contains all the values,
and other column(s) list(s) the context of the value.
In certain cases, the long-format is not really “human-readable,” and another format,
a wide-format is preferred, as a more compact way of representing the data.
This is typically the case with gene expression values that scientists are used to
look as matrices, were rows represent genes and columns represent samples.
In this format, it would become therefore straightforward to explore the relationship between the gene expression levels within, and between, the samples.
## # A tibble: 1,474 × 23
## gene GSM2545336 GSM2545337 GSM2545338 GSM2545339 GSM2545340 GSM2545341
## <chr> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
## 1 Asl 1170 361 400 586 626 988
## 2 Apod 36194 10347 9173 10620 13021 29594
## 3 Cyp2d22 4060 1616 1603 1901 2171 3349
## 4 Klk6 287 629 641 578 448 195
## 5 Fcrls 85 233 244 237 180 38
## 6 Slc2a4 782 231 248 265 313 786
## 7 Exd2 1619 2288 2235 2513 2366 1359
## 8 Gjc2 288 595 568 551 310 146
## 9 Plp1 43217 101241 96534 58354 53126 27173
## 10 Gnb4 1071 1791 1867 1430 1355 798
## # … with 1,464 more rows, and 16 more variables: GSM2545342 <dbl>,
## # GSM2545343 <dbl>, GSM2545344 <dbl>, GSM2545345 <dbl>, GSM2545346 <dbl>,
## # GSM2545347 <dbl>, GSM2545348 <dbl>, GSM2545349 <dbl>, GSM2545350 <dbl>,
## # GSM2545351 <dbl>, GSM2545352 <dbl>, GSM2545353 <dbl>, GSM2545354 <dbl>,
## # GSM2545362 <dbl>, GSM2545363 <dbl>, GSM2545380 <dbl>To convert the gene expression values from rna into a wide-format,
we need to create a new table where the values of the sample column would
become the names of column variables.
The key point here is that we are still following a tidy data structure, but we have reshaped the data according to the observations of interest: expression levels per gene instead of recording them per gene and per sample.
The opposite transformation would be to transform column names into values of a new variable.
We can do both these of transformations with two tidyr functions,
pivot_longer() and pivot_wider() (see
here for
details).
5.7.1 Pivoting the data into a wider format
Let’s first select the 3 first columns of rna and use pivot_wider()
to transform data in a wide-format.
rna_exp <- rna %>%
select(gene, sample, expression)
rna_exp## # A tibble: 32,428 × 3
## gene sample expression
## <chr> <chr> <dbl>
## 1 Asl GSM2545336 1170
## 2 Apod GSM2545336 36194
## 3 Cyp2d22 GSM2545336 4060
## 4 Klk6 GSM2545336 287
## 5 Fcrls GSM2545336 85
## 6 Slc2a4 GSM2545336 782
## 7 Exd2 GSM2545336 1619
## 8 Gjc2 GSM2545336 288
## 9 Plp1 GSM2545336 43217
## 10 Gnb4 GSM2545336 1071
## # … with 32,418 more rowspivot_wider takes three main arguments:
- the data to be transformed;
- the
names_from: the column whose values will become new column names; - the
values_from: the column whose values will fill the new columns.
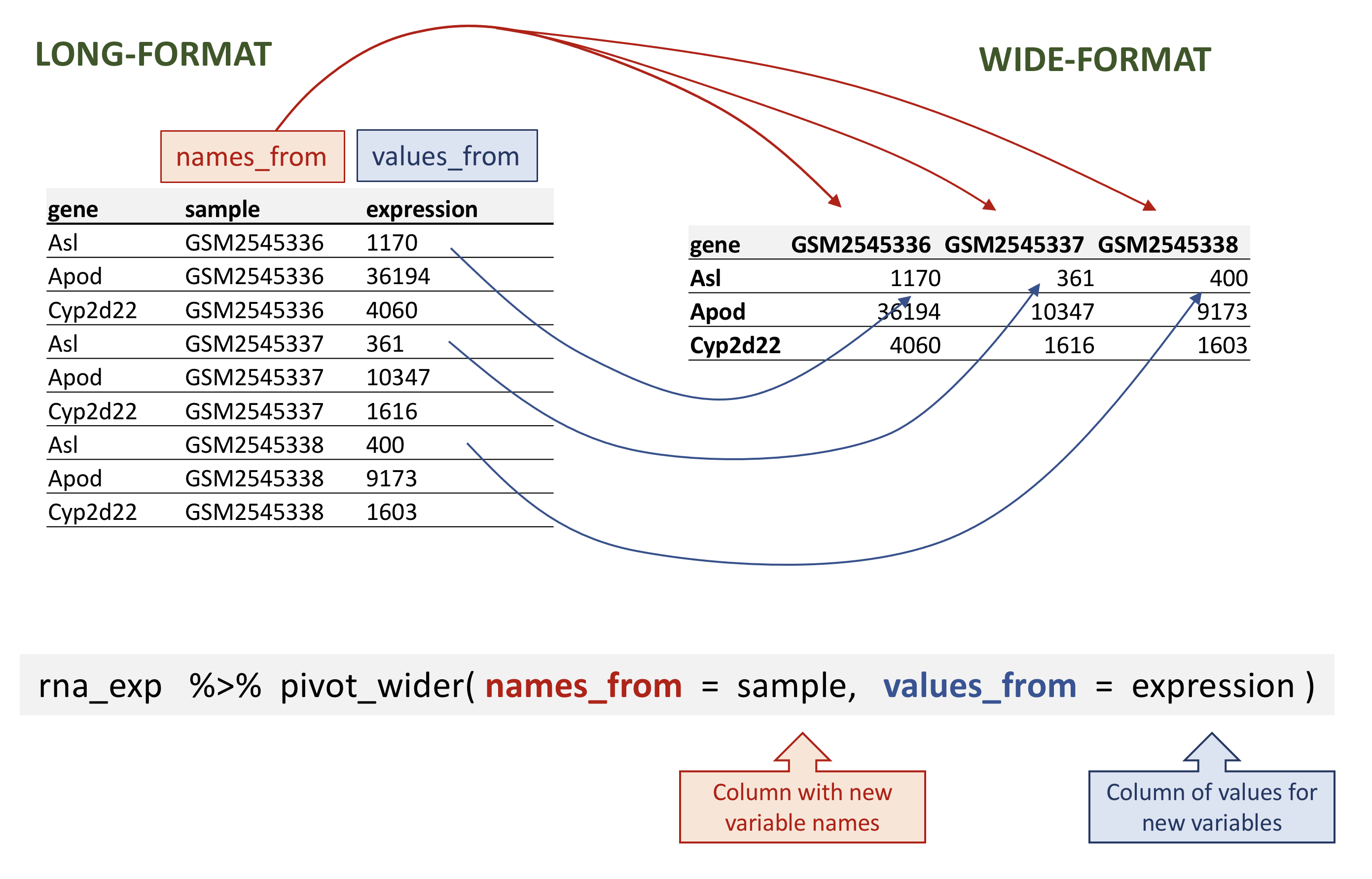
rna_wide <- rna_exp %>%
pivot_wider(names_from = sample,
values_from = expression)
rna_wide## # A tibble: 1,474 × 23
## gene GSM2545336 GSM2545337 GSM2545338 GSM2545339 GSM2545340 GSM2545341
## <chr> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
## 1 Asl 1170 361 400 586 626 988
## 2 Apod 36194 10347 9173 10620 13021 29594
## 3 Cyp2d22 4060 1616 1603 1901 2171 3349
## 4 Klk6 287 629 641 578 448 195
## 5 Fcrls 85 233 244 237 180 38
## 6 Slc2a4 782 231 248 265 313 786
## 7 Exd2 1619 2288 2235 2513 2366 1359
## 8 Gjc2 288 595 568 551 310 146
## 9 Plp1 43217 101241 96534 58354 53126 27173
## 10 Gnb4 1071 1791 1867 1430 1355 798
## # … with 1,464 more rows, and 16 more variables: GSM2545342 <dbl>,
## # GSM2545343 <dbl>, GSM2545344 <dbl>, GSM2545345 <dbl>, GSM2545346 <dbl>,
## # GSM2545347 <dbl>, GSM2545348 <dbl>, GSM2545349 <dbl>, GSM2545350 <dbl>,
## # GSM2545351 <dbl>, GSM2545352 <dbl>, GSM2545353 <dbl>, GSM2545354 <dbl>,
## # GSM2545362 <dbl>, GSM2545363 <dbl>, GSM2545380 <dbl>Note that by default, the pivot_wider() function will add NA for missing values.
Let’s imagine that for some reason, we had some missing expression values for some genes in certain samples. In the following fictive example, the gene Cyp2d22 has only one expression value, in GSM2545338 sample.
rna_with_missing_values## # A tibble: 7 × 3
## gene sample expression
## <chr> <chr> <dbl>
## 1 Asl GSM2545336 1170
## 2 Apod GSM2545336 36194
## 3 Asl GSM2545337 361
## 4 Apod GSM2545337 10347
## 5 Asl GSM2545338 400
## 6 Apod GSM2545338 9173
## 7 Cyp2d22 GSM2545338 1603By default, the pivot_wider() function will add NA for missing values.
rna_with_missing_values %>%
pivot_wider(names_from = sample,
values_from = expression)## # A tibble: 3 × 4
## gene GSM2545336 GSM2545337 GSM2545338
## <chr> <dbl> <dbl> <dbl>
## 1 Asl 1170 361 400
## 2 Apod 36194 10347 9173
## 3 Cyp2d22 NA NA 16035.7.2 Pivoting data into a longer format
In the opposite situation we are using the column names and turning them into a pair of new variables. One variable represents the column names as values, and the other variable contains the values previously associated with the column names.
pivot_longer() takes four main arguments:
- the data to be transformed;
- the
names_to: the new column name we wish to create and populate with the current column names; - the
values_to: the new column name we wish to create and populate with current values; - the names of the columns to be used to populate the
names_toandvalues_tovariables (or to drop).
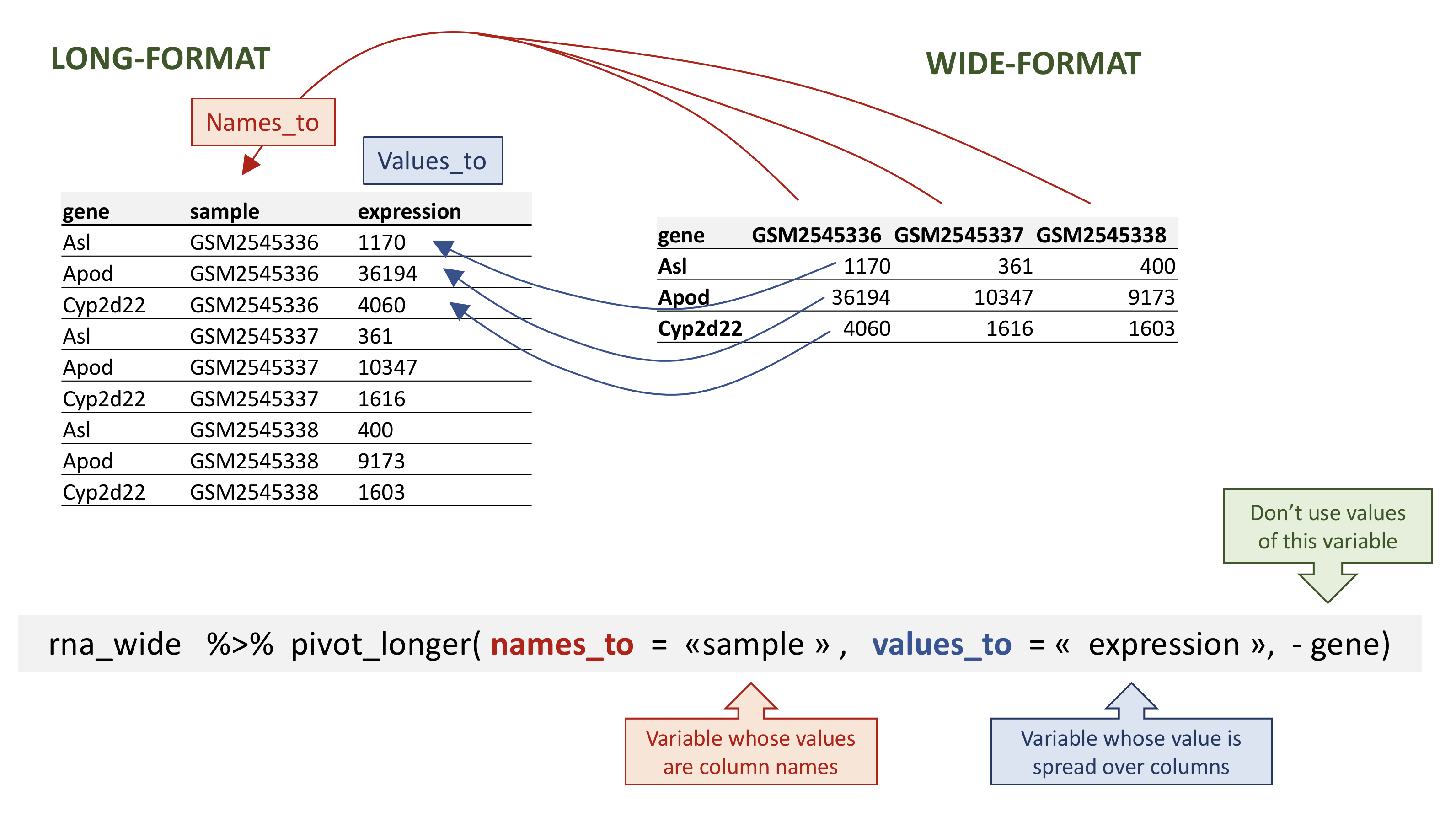
To recreate rna_long from rna_long we would create a key
called sample and value called expression and use all columns
except gene for the key variable. Here we drop gene column
with a minus sign.
Notice how the new variable names are to be quoted here.
rna_long <- rna_wide %>%
pivot_longer(names_to = "sample",
values_to = "expression",
-gene)
rna_long## # A tibble: 32,428 × 3
## gene sample expression
## <chr> <chr> <dbl>
## 1 Asl GSM2545336 1170
## 2 Asl GSM2545337 361
## 3 Asl GSM2545338 400
## 4 Asl GSM2545339 586
## 5 Asl GSM2545340 626
## 6 Asl GSM2545341 988
## 7 Asl GSM2545342 836
## 8 Asl GSM2545343 535
## 9 Asl GSM2545344 586
## 10 Asl GSM2545345 597
## # … with 32,418 more rowsWe could also have used a specification for what columns to
include. This can be useful if you have a large number of identifying
columns, and it’s easier to specify what to gather than what to leave
alone. Here the starts_with() function can help to retrieve sample
names without having to list them all!
Another possibility would be to use the : operator!
rna_wide %>%
pivot_longer(names_to = "sample",
values_to = "expression",
cols = starts_with("GSM"))## # A tibble: 32,428 × 3
## gene sample expression
## <chr> <chr> <dbl>
## 1 Asl GSM2545336 1170
## 2 Asl GSM2545337 361
## 3 Asl GSM2545338 400
## 4 Asl GSM2545339 586
## 5 Asl GSM2545340 626
## 6 Asl GSM2545341 988
## 7 Asl GSM2545342 836
## 8 Asl GSM2545343 535
## 9 Asl GSM2545344 586
## 10 Asl GSM2545345 597
## # … with 32,418 more rowsrna_wide %>%
pivot_longer(names_to = "sample",
values_to = "expression",
GSM2545336:GSM2545380)## # A tibble: 32,428 × 3
## gene sample expression
## <chr> <chr> <dbl>
## 1 Asl GSM2545336 1170
## 2 Asl GSM2545337 361
## 3 Asl GSM2545338 400
## 4 Asl GSM2545339 586
## 5 Asl GSM2545340 626
## 6 Asl GSM2545341 988
## 7 Asl GSM2545342 836
## 8 Asl GSM2545343 535
## 9 Asl GSM2545344 586
## 10 Asl GSM2545345 597
## # … with 32,418 more rowsNote that if we had missing values in the wide-format, the NA would be
included in the new long format.
Remember our previous fictive tibble containing missing values:
rna_with_missing_values## # A tibble: 7 × 3
## gene sample expression
## <chr> <chr> <dbl>
## 1 Asl GSM2545336 1170
## 2 Apod GSM2545336 36194
## 3 Asl GSM2545337 361
## 4 Apod GSM2545337 10347
## 5 Asl GSM2545338 400
## 6 Apod GSM2545338 9173
## 7 Cyp2d22 GSM2545338 1603wide_with_NA <- rna_with_missing_values %>%
pivot_wider(names_from = sample,
values_from = expression)
wide_with_NA## # A tibble: 3 × 4
## gene GSM2545336 GSM2545337 GSM2545338
## <chr> <dbl> <dbl> <dbl>
## 1 Asl 1170 361 400
## 2 Apod 36194 10347 9173
## 3 Cyp2d22 NA NA 1603wide_with_NA %>%
pivot_longer(names_to = "sample",
values_to = "expression",
-gene)## # A tibble: 9 × 3
## gene sample expression
## <chr> <chr> <dbl>
## 1 Asl GSM2545336 1170
## 2 Asl GSM2545337 361
## 3 Asl GSM2545338 400
## 4 Apod GSM2545336 36194
## 5 Apod GSM2545337 10347
## 6 Apod GSM2545338 9173
## 7 Cyp2d22 GSM2545336 NA
## 8 Cyp2d22 GSM2545337 NA
## 9 Cyp2d22 GSM2545338 1603Pivoting to wider and longer formats can be a useful way to balance out a dataset so every replicate has the same composition.
► Question
Subset genes located on X and Y chromosomes from the rna data frame and
spread the data frame with sex as columns, chromosome_name as
rows, and the mean expression of genes located in each chromosome as the values,
as in the following tibble:
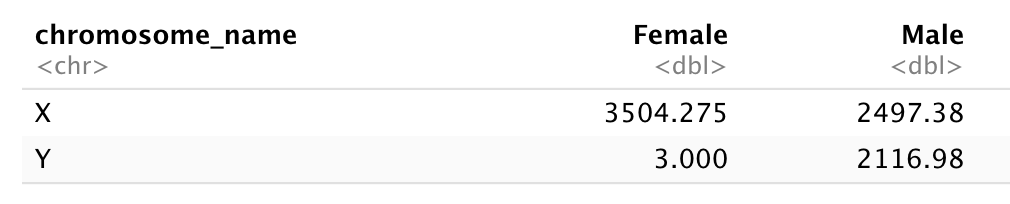
You will need to summarize before reshaping!
► Solution
► Question
Now take that data frame and transform it with pivot_longer() so
each row is a unique chromosome_name by gender combination.
► Solution
► Question
Use the rna dataset to create an expression matrix were each row represents
the mean expression levels of genes and columns represent the different timepoints.
► Solution
► Question
Use the previous data frame containing mean expression levels per timepoint and create a new column containing fold-changes between timepoint 8 and timepoint 0, and fold-changes between timepoint 8 and timepoint 4. Convert this table in a long-format table gathering the foldchanges calculated.
► Solution
5.8 Exporting data
Now that you have learned how to use dplyr to extract information from
or summarize your raw data, you may want to export these new data sets to share
them with your collaborators or for archival.
Similar to the read_csv() function used for reading CSV files into R, there is
a write_csv() function that generates CSV files from data frames.
Before using write_csv(), we are going to create a new folder, data_output,
in our working directory that will store this generated dataset. We don’t want
to write generated datasets in the same directory as our raw data.
It’s good practice to keep them separate. The data folder should only contain
the raw, unaltered data, and should be left alone to make sure we don’t delete
or modify it. In contrast, our script will generate the contents of the data_output
directory, so even if the files it contains are deleted, we can always
re-generate them.
Let’s use write_csv() to save the rna_wide table that we have created previously.
write_csv(rna_wide, file = "data_output/rna_wide.csv")Page built: 2021-10-01 using R version 4.1.1 (2021-08-10)