scplainer: reanalysis of the AML dataset (Schoof et al. 2021)
Christophe Vanderaa1, Computational Biology, UCLouvain
Laurent Gatto, Computational Biology, UCLouvain
8 December 2023
scplainer_schoof2021.RmdIntroduction
In this vignette, we will analyse the schoof2021 dataset
using the scplainer approach. The data were acquired using the
SCoPE2-derived acquisition protocol (Schoof et
al. 2021). Single cells were labelled with TMT-16 and MS data were
acquired in DDA mode. The objective of the study was to show that mass
spectrometry-based single-cell proteomics can effectively characterised
the hierarchy of cellular differentiation in an accute myleoid leukemia
model (AML).
Packages and data
We rely on several packages to compile this vignette.
## Core packages
library("scp")
library("scpdata")
library("SingleCellExperiment")
## Utility packages
library("ggplot2")
library("patchwork")
library("dplyr")
library("ensembldb")
library("EnsDb.Hsapiens.v86")
library("destiny")
library("scater")
library("plotly")The data set is available from the scpdata package.
schoof <- schoof2021()
## see ?scpdata and browseVignettes('scpdata') for documentation
## loading from cacheThe data set consists of four cell types as identied by upstream flow cytometry: leukemic stem cells (LSC), progenitor cells (PROG), CD38 positive blast cells (BLAST CD38+) and CD38 negative blast cells (BLAST CD38-).
table(schoof$Population)
##
## BLAST CD38- BLAST CD38+ LSC PROG
## 1747 589 93 19The dataset contains booster samples (also called carrier samples), empty wells, negative control samples, normalisation samples and single cells.
table(schoof$SampleType)
##
## booster_1:1:1 booster_bulk empty neg control norm
## 72 120 192 48 192
## sc
## 2448The data were acquired using TMT-16 labelling.
table(schoof$Channel)
##
## 126 127N 127C 128N 128C 129N 129C 130N 130C 131N 131C 132N 132C 133N 133C 134N
## 192 192 192 192 192 192 192 192 192 192 192 192 192 192 192 192The data were acquired as part of 192 MS acquisition batches.
Finally, samples were prepared through 8 sample preparation batches performed in 384-well plates.
table(schoof$Plate)
##
## bulk_b_1 bulk_b_2 bulk_b_3 bulk_b_e_1 bulk_b_e_2 bulk_c_1 bulk_c_2
## 384 384 384 384 384 384 384
## bulk_c_3
## 384Minimal data processing
The minimal data processing workflow in scplainer consists of 5 main steps:
- Data cleaning
- Feature quality control
- Sample quality control
- Peptide data assembly
- Log2-transformation
Cleaning data
We remove the protein assays that were processed by the authors. The vignette only uses the PTM data generated by Proteome Discoverer.
schoof <- removeAssay(schoof, c("proteins", "logNormProteins"))
## Warning: 'experiments' dropped; see 'drops()'
## harmonizing input:
## removing 5097 sampleMap rows not in names(experiments)We remove feature annotations that won’t be used in the remainder of the vignette. This is to avoid overcrowding of the annotation tables later in the vignette.
requiredRowData <- c(
"Annotated.Sequence", "isContaminant",
"Master.Protein.Accessions", "Isolation.Interference.in.Percent",
"Percolator.q.Value"
)
schoof <- selectRowData(schoof, requiredRowData)We replace zeros by missing values. A zero may be a true (the feature
is not present in the sample) or because of technical limitations (due
to the technology or the computational pre-processing). Because we are
not able to distinguish between the two, zeros should be replaced with
NA.
Feature quality control
We remove low-quality PSMs that may propagate technical artefacts and bias data modelling. The quality control criteria are:
- We remove contaminants and decoy peptides.
- We remove PSMs with low spectral purity.
- We remove low-confidence peptides, as defined by the false discovery rate (FDR) computed by Percolator.
- We remove PSMs for which the signal in single-cell samples exceeds 5% of the signal in 100-cell samples, i.e. the carrier.
All the criteria were readily computed except for the
sample-to-carrier ratio. We compute this using
computeSCR(). The results are stored in the
rowData.
schoof <- computeSCR(
schoof, names(schoof), colvar = "SampleType",
samplePattern = "sc", carrierPattern = "^boost",
sampleFUN = "mean", rowDataName = "MeanSCR"
)Here is an overview of the distributions of each criteria
## `stat_bin()` using `bins = 30`. Pick better value with `binwidth`.
## `stat_bin()` using `bins = 30`. Pick better value with `binwidth`.
## `stat_bin()` using `bins = 30`. Pick better value with `binwidth`.
## Warning: Removed 25437 rows containing non-finite values (`stat_bin()`).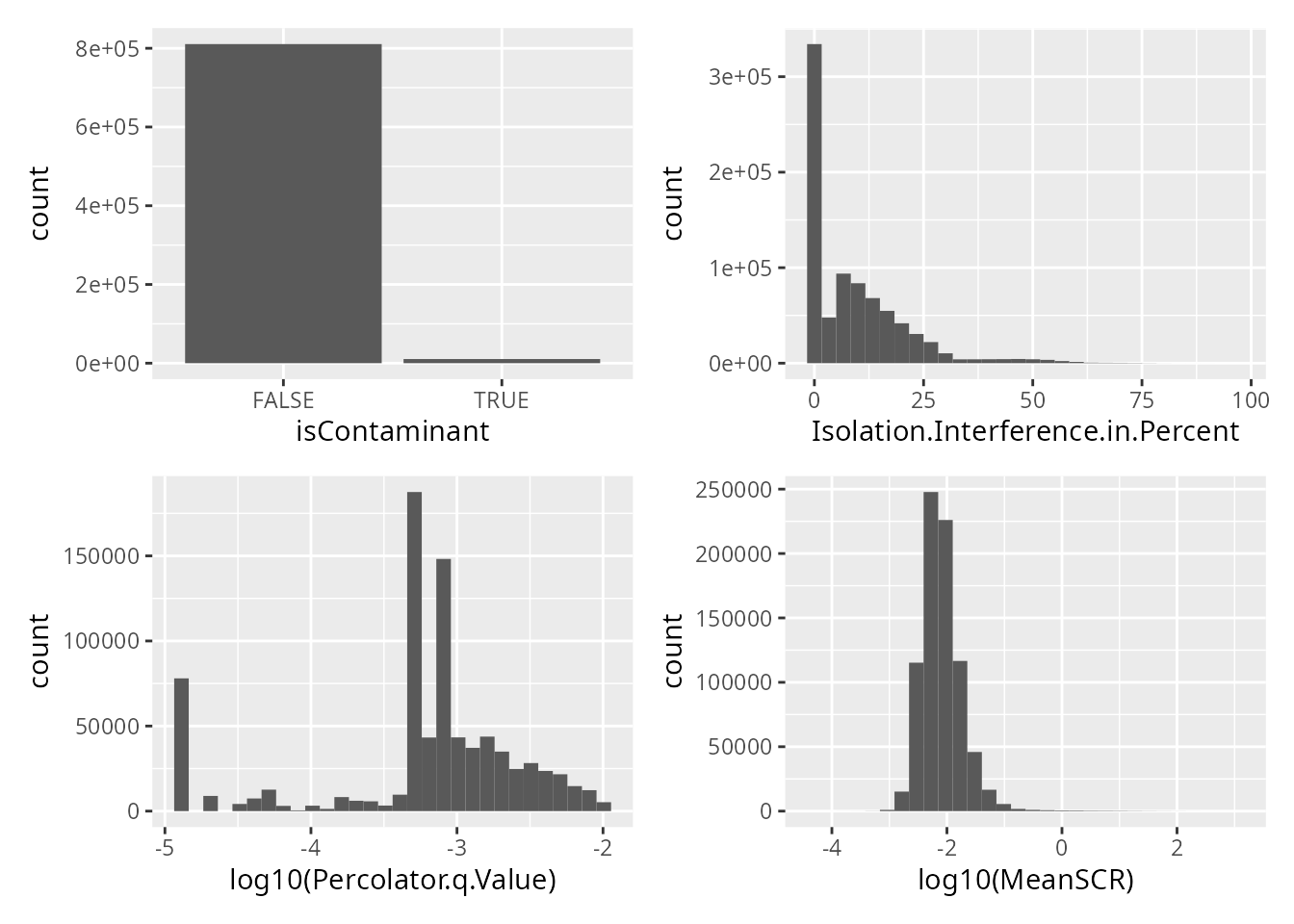
We filter and keep the features that pass the quality control criteria.
schoof <- filterFeatures(
schoof, ~ Percolator.q.Value < 0.01 &
!isContaminant & Master.Protein.Accessions != "" &
Isolation.Interference.in.Percent < 30 &
!is.na(MeanSCR) & MeanSCR < 0.2
)
## 'Percolator.q.Value' found in 192 out of 192 assay(s)
## 'isContaminant' found in 192 out of 192 assay(s)
## 'Master.Protein.Accessions' found in 192 out of 192 assay(s)
## 'Isolation.Interference.in.Percent' found in 192 out of 192 assay(s)
## 'MeanSCR' found in 192 out of 192 assay(s)Sample quality control
Similarly to the features, we also remove low-quality cells. The quality control criteria are:
- We remove samples with a low number of detected peptides. The criterion is computed as follows:
schoof <- countUniqueFeatures(
schoof, i = names(schoof), groupBy = "Annotated.Sequence",
colDataName = "NumberPeptides"
)- We remove samples with low median intensity. The metric (note we will later use it for normalisation) is computed as follows:
MedianIntensity <- lapply(experiments(schoof), function(x) {
out <- colMedians(log(assay(x)), na.rm = TRUE)
names(out) <- colnames(x)
out
})
names(MedianIntensity) <- NULL
MedianIntensity <- unlist(MedianIntensity)
colData(schoof)[names(MedianIntensity), "MedianIntensity"] <- MedianIntensity- We remove the samples that have a high median coefficient of variation (CV). The CV is computed within each sample, by grouping the peptides that belong to the same protein or protein group. This is computed as follows:
schoof <- medianCVperCell(
schoof, i = names(schoof), groupBy = "Master.Protein.Accessions",
nobs = 3, na.rm = TRUE, colDataName = "MedianCV", norm = "SCoPE2"
)
## Warning in medianCVperCell(schoof, i = names(schoof), groupBy = "Master.Protein.Accessions", : The median CV could not be computed for one or more samples. You may want to try a smaller value for 'nobs'.- We also remove the samples that are not single cells because we will no longer need them.
We plot the metrics used to perform sample quality control.
ggplot(data.frame(colData(schoof))) +
aes(
y = MedianIntensity,
x = NumberPeptides,
color = MedianCV,
shape = SampleType
) +
geom_point(size = 2) +
scale_color_continuous(type = "viridis")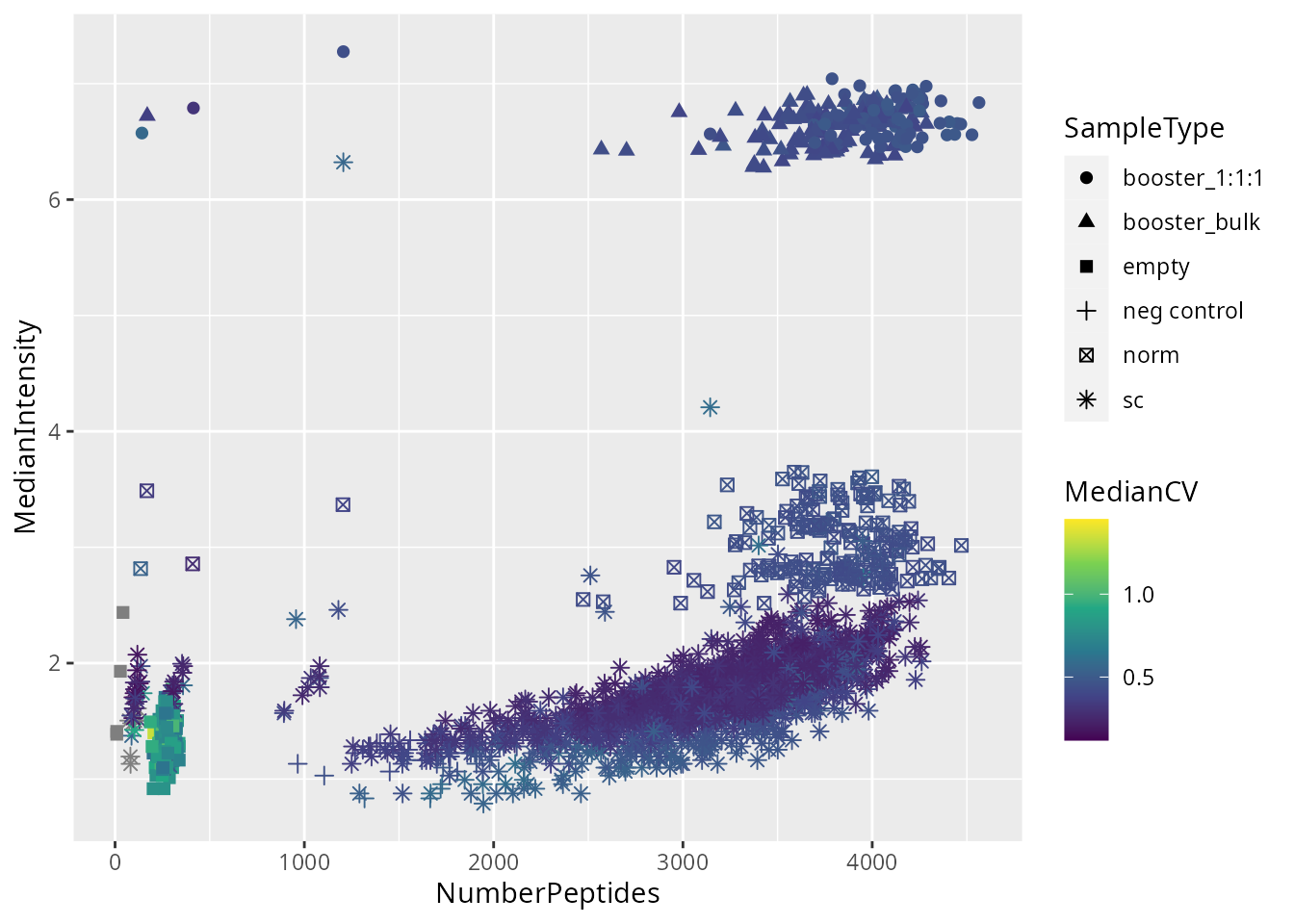
We apply the filter and keep only single cells that pass the quality control.
passQC <- !is.na(schoof$MedianCV) & schoof$MedianCV < 0.4 &
schoof$MedianIntensity < 3 &
schoof$NumberPeptides > 1200 &
schoof$SampleType == "sc"
schoof <- subsetByColData(schoof, passQC)
schoof <- dropEmptyAssays(schoof)Peptide data assembly
For now, each MS acquisition run is stored separately in an assay. We here combine these assays in one. The issue is that PSMs are specific to each run. We therefore aggregate the PSMs into peptides.
peptideAssays <- paste0("peptides_", names(schoof))
schoof <- aggregateFeatures(
schoof,
i = names(schoof),
fcol = "Annotated.Sequence",
name = peptideAssays,
fun = colMedians,
na.rm = TRUE
)The data can now be joined.
schoof <- joinAssays(schoof, i = peptideAssays, name = "peptides")Finally, we also convert Uniprot protein identifiers to gene symbols.
proteinIds <- rowData(schoof)[["peptides"]][, "Master.Protein.Accessions"]
## Unlist protein groups
proteinIds <- unlist(sapply(proteinIds, function(x) {
strsplit(x, "; ")[[1]]
}, USE.NAMES = FALSE))
## Rename splice isoform number to canonical protein
proteinIds <- unique(sub("[-]\\d*$", "", proteinIds))
## Convert uniprot IDs to gene names
convert <- transcripts(
EnsDb.Hsapiens.v86,
columns = "gene_name",
return.type = "data.frame",
filter = UniprotFilter(proteinIds)
)
## Convert the protein groups into gene group
geneNames <- sapply(rowData(schoof)[["peptides"]]$Master.Protein.Accessions, function(x) {
out <- strsplit(x, "; ")[[1]]
out <- sapply(out, function(xx) {
gene <- convert$gene_name[convert$uniprot_id == sub("[-]\\d*$", "", xx)]
if (!length(gene)) return(NA)
unique(gene)
})
paste(out, collapse = "; ")
})
rowData(schoof)[["peptides"]]$Gene <- unname(geneNames)
head(rowData(schoof)[["peptides"]][, "Master.Protein.Accessions"])
## [1] "P05109" "P13639" "P60709; Q6S8J3; P68133"
## [4] "P19338" "P62249" "P29401"
head(rowData(schoof)[["peptides"]][, "Gene"])
## [1] "S100A8" "EEF2" "ACTB; POTEE; ACTA1"
## [4] "NCL" "RPS16" "TKT"Log-transformation
We log2-transform the quantification data.
schoof <- logTransform(schoof, i = "peptides", name = "peptides_log")Here is an overview of the data processing:
plot(schoof)
## Warning in plot.QFeatures(schoof): The QFeatures object contains many assays.
## You may want to consider creating an interactive plot (set 'interactive =
## TRUE')
Data modelling
Model the data using scplainer, the linear regression model
implemented in scp. scplainer is applied on a
SingleCellExperiment so we extract it from the processed
data set along with the colData
sce <- getWithColData(schoof, "peptides_log")First, we must specify which variables to include in the model. We here include 4 variables:
-
MedianIntensity: this is the normalisation factor used to correct for cell-specific technical differences. -
Channel: this is used to correct for TMT effects. -
File.ID: this is used to perform batch correction. We consider each acquisition run to be a batch. -
Population: this is the biological variable of interest. It captures the difference between the different AML populations
scpModelWorkflow() fits linear regression models to the
data, where the model is adapted for each peptide depending on its
pattern of missing values.
sce <- scpModelWorkflow(
sce,
formula = ~ 1 + ## intercept
## normalisation
MedianIntensity +
## batch effects
Channel + File.ID +
## biological variability
Population
)Once the models are fit, we can explore the distribution of the n/p ratios.
scpModelFilterThreshold(sce) <- 3
scpModelFilterPlot(sce)
## To change the threshold, use:
## scpModelFilterThreshold(object, name) <- threshold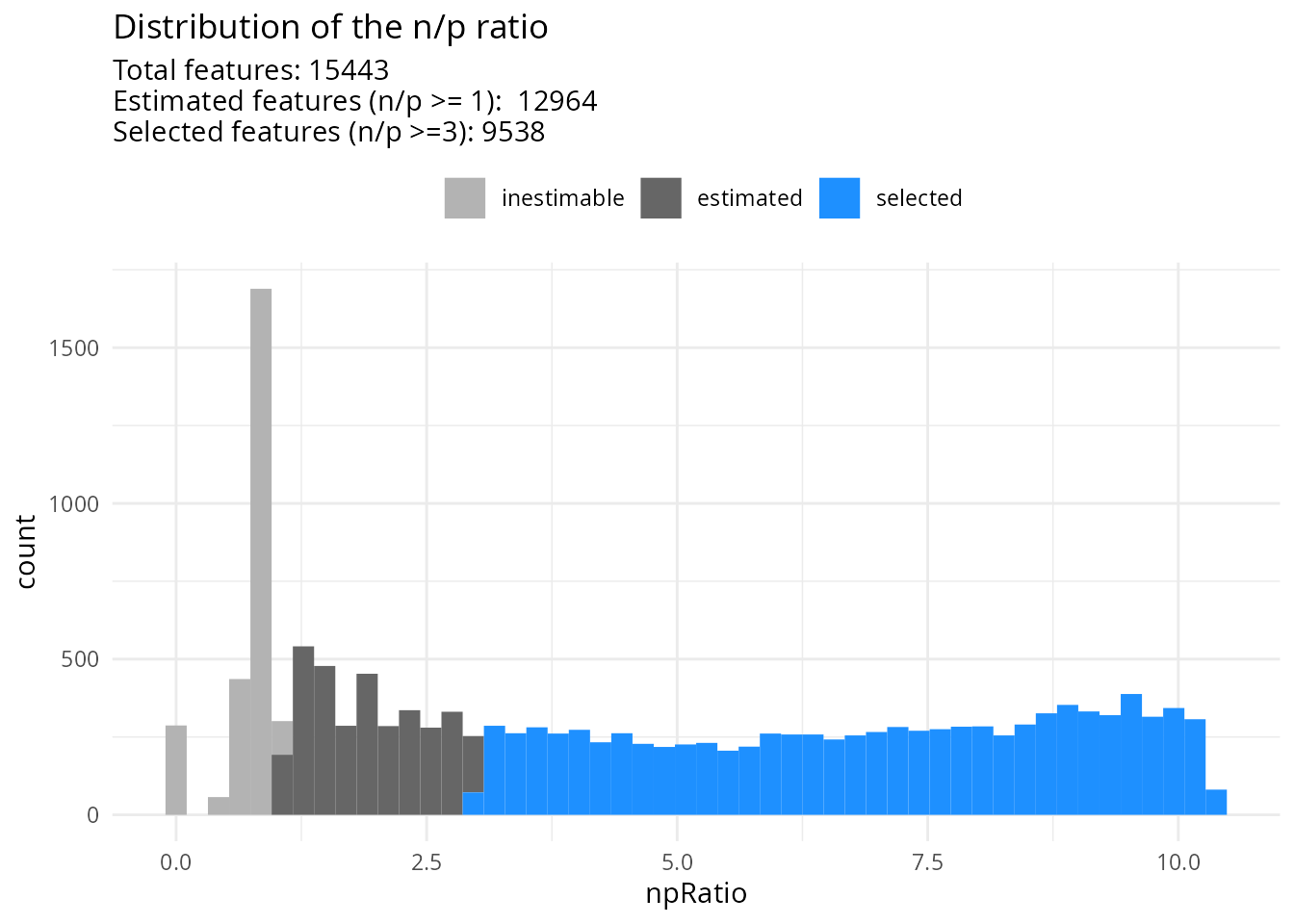
Many peptides do not have sufficient observations to estimate the model. We have chosen to continue the analysis with peptides that have \(n/p >= 3\). You could consider \(n/p\) a rough average of the number of replicates per parameter to fit (for categorical variables, the number of replicates per group). We recommend moving the threshold away from 1 to increase statistical power and remove noisy peptides. This comes of course at the cost of less peptides included in the analysis.
Model analysis
The model analysis consists of three steps:
- Analysis of variance
- Differential abundance analysis
- Component analysis
Analysis of variance
The analysis of variance explores the proportion of data captures by each variable in the model.
(vaRes <- scpVarianceAnalysis(sce))
## DataFrameList of length 5
## names(5): Residuals MedianIntensity Channel File.ID Population
vaRes[[1]]
## DataFrame with 9538 rows and 4 columns
## feature SS df percentExplainedVar
## <character> <numeric> <numeric> <numeric>
## 1 [-].mLTELE... 2578.954 1887 68.1597
## 2 [-].mVNFTV... 329.300 1889 22.1701
## 3 [K].aGFAGD... 519.815 1892 39.3162
## 4 [K].aLELTG... 554.172 1750 17.8839
## 5 [K].aLVAYY... 399.764 1624 22.4221
## ... ... ... ... ...
## 9534 [R].eVQTAV... 45.2557 202 47.4447
## 9535 [R].iDEYDY... 52.8887 330 36.1397
## 9536 [R].tVVSGL... 29.9509 163 46.7577
## 9537 [K].aPQVVA... 15.3313 77 24.3418
## 9538 [K].aVTTPG... 27.0801 83 18.8546The results are a list of tables, one table for each variable. Each
table reports for each peptide the variance captures (SS),
the residual degrees of freedom for estimating the variance
(df) and the percentage of total variance explained
(percentExplainedVar). To better explore the results, we
add the annotations available in the rowData.
vaRes <- scpAnnotateResults(
vaRes, rowData(sce), by = "feature", by2 = "Annotated.Sequence"
)By default, we explore the variance for all peptides combined:
scpVariancePlot(vaRes)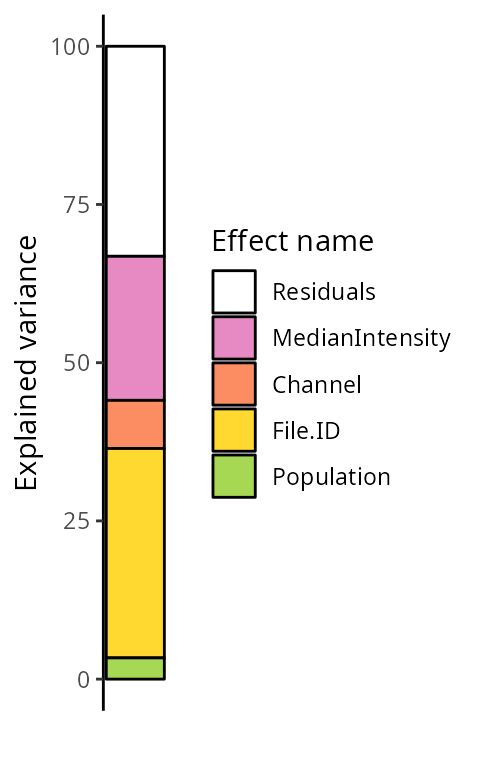
We explore the top 20 peptides that are have the highest percentage of variance explained by the biological variable (top) or by the batch variable (bottom).
scpVariancePlot(
vaRes, top = 20, by = "percentExplainedVar", effect = "Population",
decreasing = TRUE, combined = FALSE
) +
scpVariancePlot(
vaRes, top = 20, by = "percentExplainedVar", effect = "File.ID",
decreasing = TRUE, combined = FALSE
) +
plot_layout(ncol = 1, guides = "collect")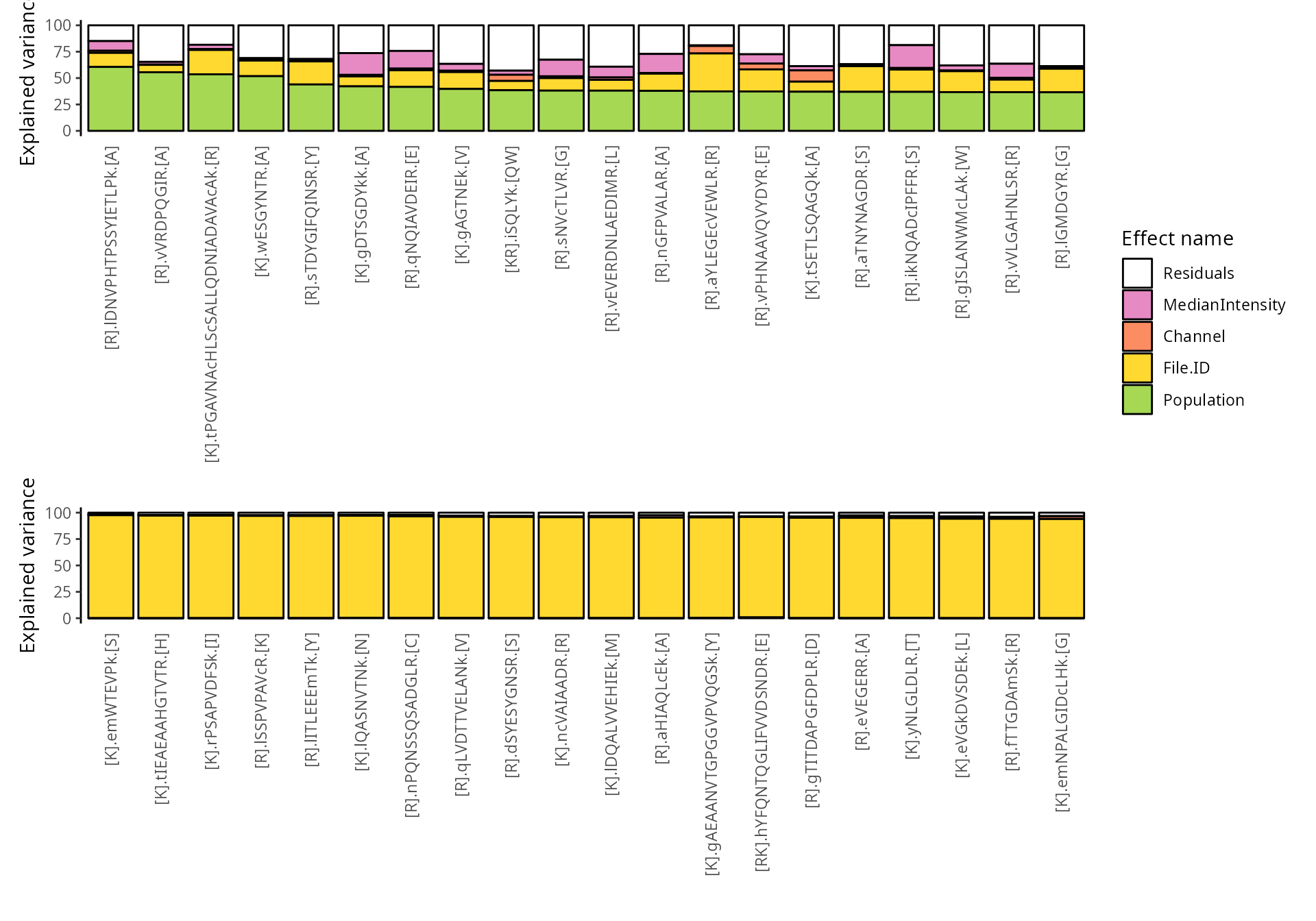
We can also group these peptide according to their protein. We simply
need to provide the fcol argument.
scpVariancePlot(
vaRes, top = 20, by = "percentExplainedVar", effect = "Population",
decreasing = TRUE, combined = FALSE, fcol = "Gene"
) +
scpVariancePlot(
vaRes, top = 20, by = "percentExplainedVar", effect = "File.ID",
decreasing = TRUE, combined = FALSE, fcol = "Gene"
) +
plot_layout(ncol = 1, guides = "collect")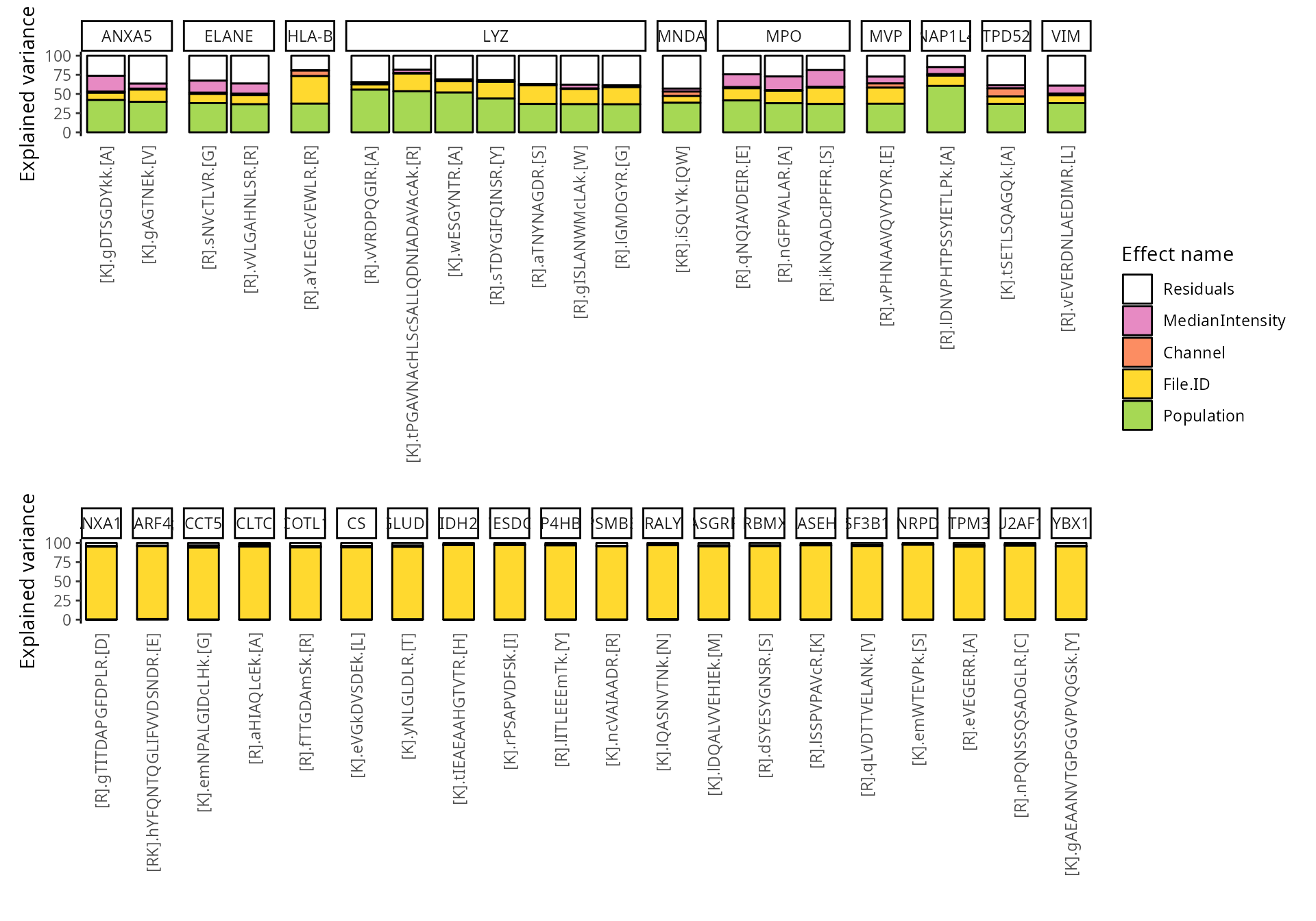
Differential abundance analysis
Next, we explore the model output to understand the differences
between melanoma cells and monocytes. The difference of interest is
specified using the contrast argument. The first element
points to the variable to test and the two following element are the
groups of interest to compare. You can provide multiple contrast in a
list.
(daRes <- scpDifferentialAnalysis(
sce, contrast = list(
c("Population", "BLAST CD38-", "LSC"),
c("Population", "BLAST CD38-", "PROG"),
c("Population", "BLAST CD38-", "BLAST CD38+")
)
))
## List of length 3
## names(3): Population_BLAST.CD38._vs_LSC ...
daRes[[1]]
## DataFrame with 9538 rows and 7 columns
## feature Estimate SE Df tstatistic pvalue
## <character> <numeric> <numeric> <numeric> <numeric> <numeric>
## 1 [-].mLTELE... 0.872374 0.1674061 1887 5.21112 2.08235e-07
## 2 [-].mVNFTV... -0.293451 0.0596068 1889 -4.92311 9.25829e-07
## 3 [K].aGFAGD... 0.242921 0.0748311 1892 3.24625 1.18980e-03
## 4 [K].aLELTG... -0.658893 0.0807045 1750 -8.16426 6.11884e-16
## 5 [K].aLVAYY... -0.268741 0.0734681 1624 -3.65792 2.62383e-04
## ... ... ... ... ... ... ...
## 9534 [R].eVQTAV... -0.1161121 0.145581 202 -0.7975772 0.426052446
## 9535 [R].iDEYDY... -0.3861179 0.111675 330 -3.4575012 0.000616609
## 9536 [R].tVVSGL... -0.4482902 0.206489 163 -2.1710103 0.031376758
## 9537 [K].aPQVVA... 0.0184523 0.205896 77 0.0896193 0.928822476
## 9538 [K].aVTTPG... -0.5251155 0.347445 83 -1.5113631 0.134494091
## padj
## <numeric>
## 1 4.48613e-06
## 2 1.72834e-05
## 3 8.88792e-03
## 4 5.53726e-14
## 5 2.47584e-03
## ... ...
## 9534 0.70455105
## 9535 0.00508154
## 9536 0.12649213
## 9537 0.97715580
## 9538 0.35928109Similarly to analysis of variance, the results are a list of tables,
one table for each contrast. Each table reports for each peptide the
estimated difference between the two groups, the standard error
associated to the estimation, the degrees of freedom, the t-statistics,
the associated p-value and the p-value FDR-adjusted for multiple testing
across all peptides. Again, to better explore the results, we add the
annotations available in the rowData.
daRes <- scpAnnotateResults(
daRes, rowData(sce),
by = "feature", by2 = "Annotated.Sequence"
)We then visualize the results using a volcano plot. The function below return a volcano plot for each contrast.
wrap_plots(scpVolcanoPlot(daRes))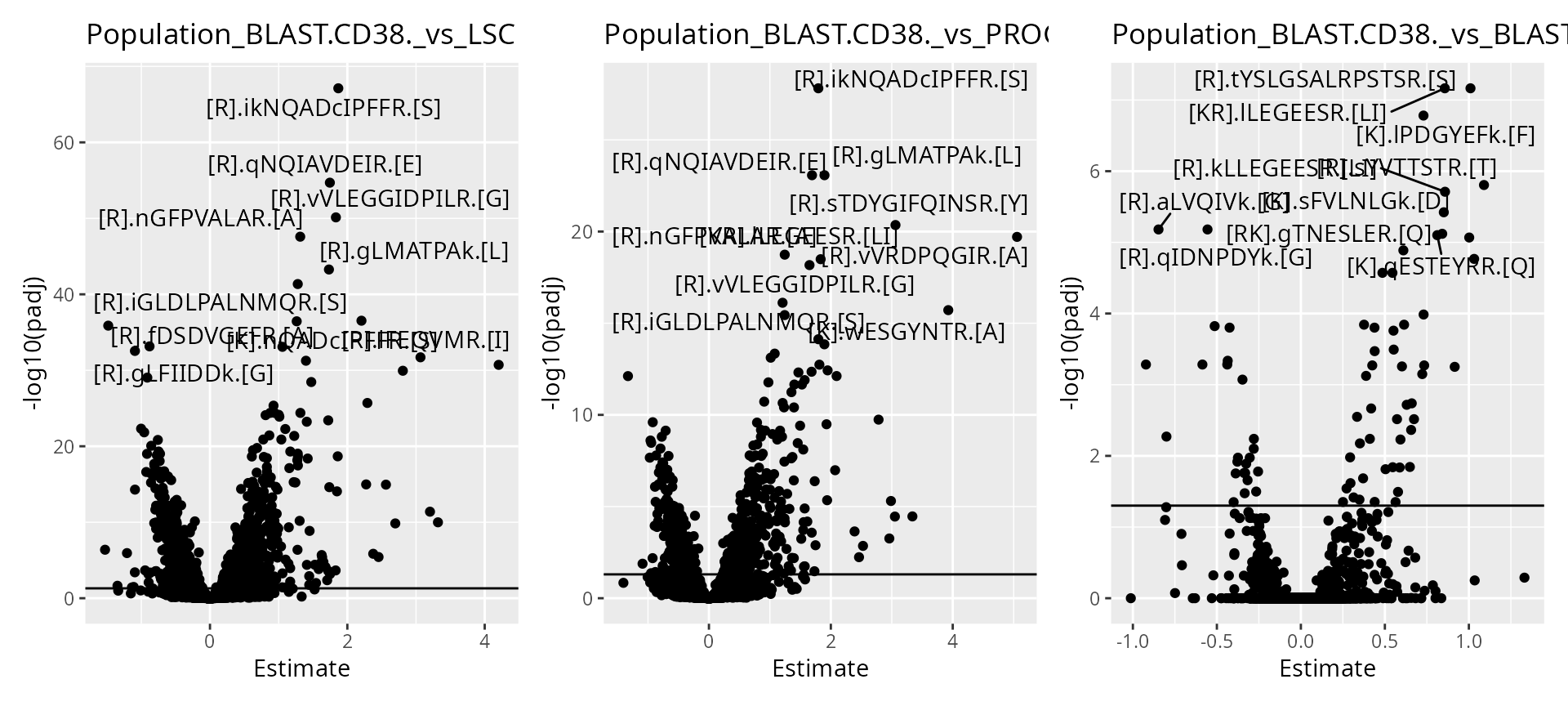
To help interpretation of the results, we will label the peptides with their gene name. Also we increase the number of labels shown on the plot. Finally, we can add colors to the plot. For instance, let’s explore the impact of the number of observations using the \(n/p\) ratio. We create a new annotation table, add it to the results and redraw the plot. The \(n/p\) ratio is retrieved using scpModelFilterNPRatio
np <- scpModelFilterNPRatio(sce)
daRes <- scpAnnotateResults(
daRes, data.frame(feature = names(np), npRatio = np),
by = "feature"
)
wrap_plots(scpVolcanoPlot(
daRes, top = 30, textBy = "Gene",
pointParams = list(aes(colour = npRatio))
))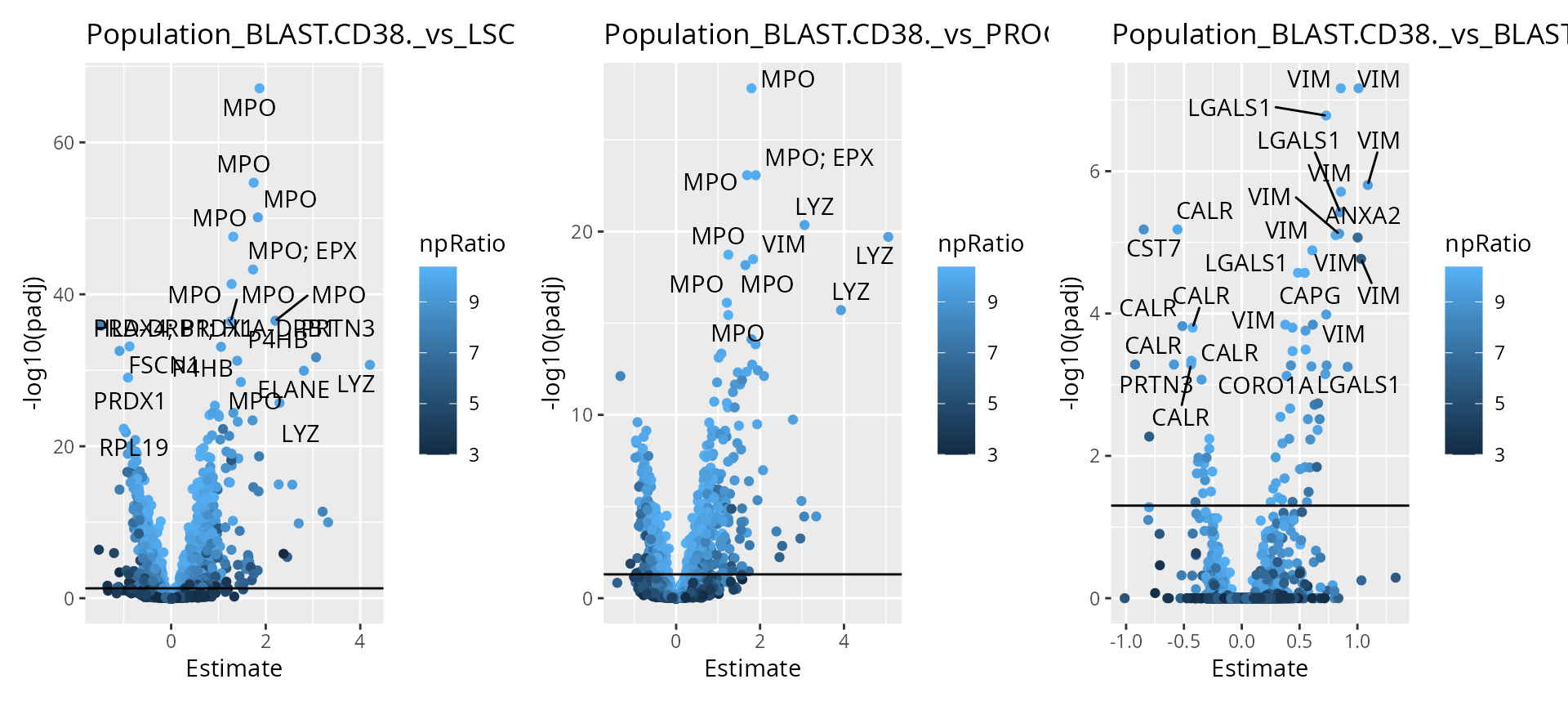
As expected, higher number of observations (higher \(n/p\)) lead to increased statistical power and hence to more significant results.
Finally, we offer functionality to report results at the protein level.
scpDifferentialAggregate(daRes, fcol = "Gene") |>
scpVolcanoPlot(top = 30) |>
wrap_plots()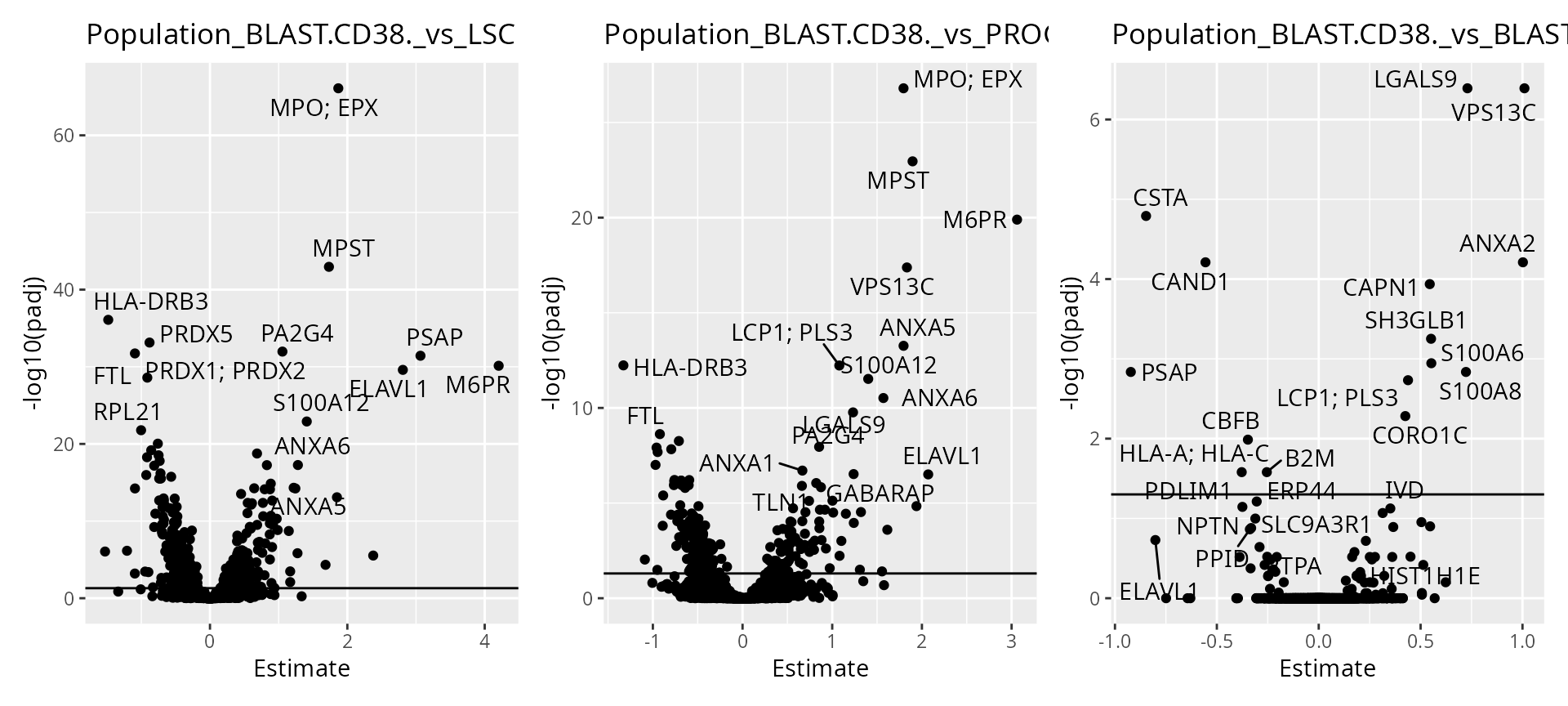
Component analysis
Finally, we perform component analysis to link the modelled effects to the cellular heterogeneity. We here run an APCA+ (extended ANOVA-simultaneous principal component analysis) for the sample type effect. In other words, we perform a PCA on the data that is capture by the sample type variable along with the residuals (unmodelled data).
(caRes <- scpComponentAnalysis(
sce, ncomp = 2, method = "APCA", effect = "Population"
))
## [1] "APCA"
## [1] "Population"
## List of length 2
## names(2): bySample byFeatureThe results are contained in a list with 2 elements.
bySample contains the PC scores, that is the component
results in sample space. byFeature contains the
eigenvectors, that is the component results in feature space.
caRes$bySample
## List of length 3
## names(3): unmodelled residuals APCA_PopulationEach of the two elements contains components results for the data
before modelling (unmodelled), for the residuals or for the
APCA on the sample type variable (APCA_Population).
caRes$bySample[[1]]
## DataFrame with 2118 rows and 3 columns
## PC1 PC2 cell
## <numeric> <numeric> <character>
## F1129N -33.213784 -5.56117 F1129N
## F1129C 22.218813 -5.76867 F1129C
## F1130N 6.103797 -19.36936 F1130N
## F1130C -13.577222 -5.21993 F1130C
## F1131N 0.991159 -5.26628 F1131N
## ... ... ... ...
## F99132N 57.95420 8.32955 F99132N
## F99132C -47.79824 -9.37227 F99132C
## F99133N -6.75363 -12.73044 F99133N
## F99133C 31.14581 14.50989 F99133C
## F99134N -15.61145 -3.46888 F99134NEach elements is a table with the computed componoents. Let’s explore the component analysis results in cell space. Similarly to the previous explorations, we annotate the results.
caResCells <- caRes$bySample
sce$cell <- colnames(sce)
caResCells <- scpAnnotateResults(caResCells, colData(sce), by = "cell")We then generate the component plot, colouring by
Population.
scpComponentPlot(
caResCells,
pointParams = list(aes(colour = Population), alpha = 0.5)
) |>
wrap_plots() +
plot_layout(guides = "collect")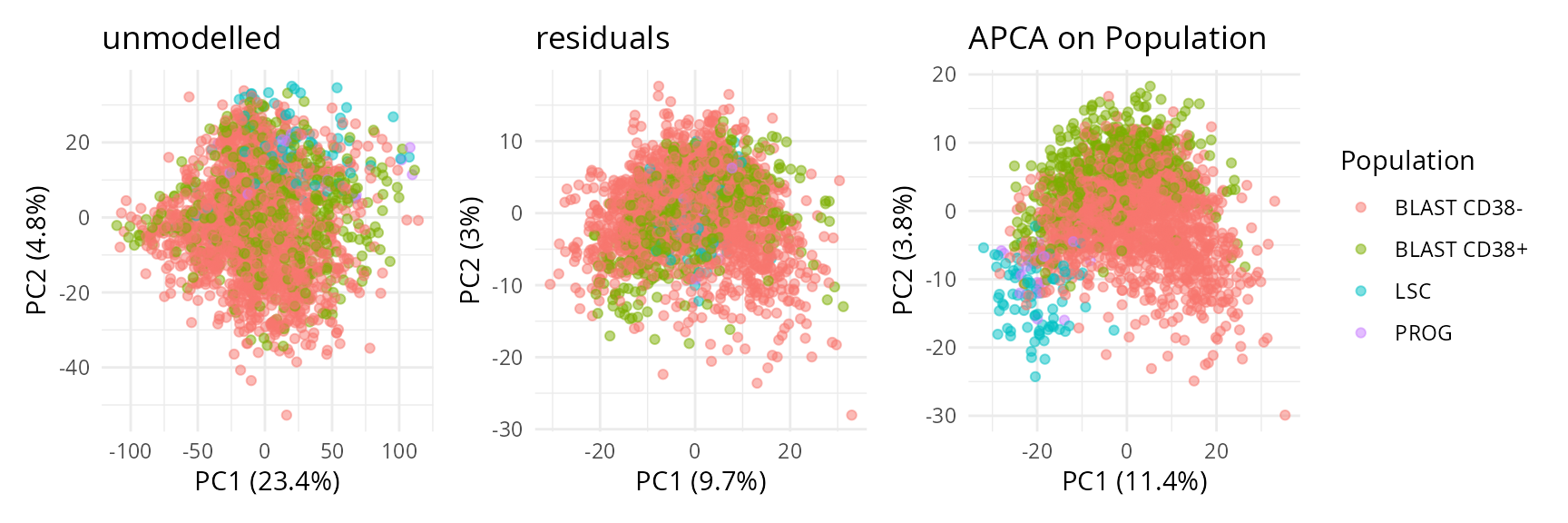
To assess the impact of batch effects, we shape the points according to the plate batch (cf intro) as well.
scpComponentPlot(
caResCells,
pointParams = list(aes(colour = as.numeric(File.ID)), alpha = 0.5)
) |>
wrap_plots() +
plot_layout(guides = "collect") &
scale_color_continuous(type = "viridis")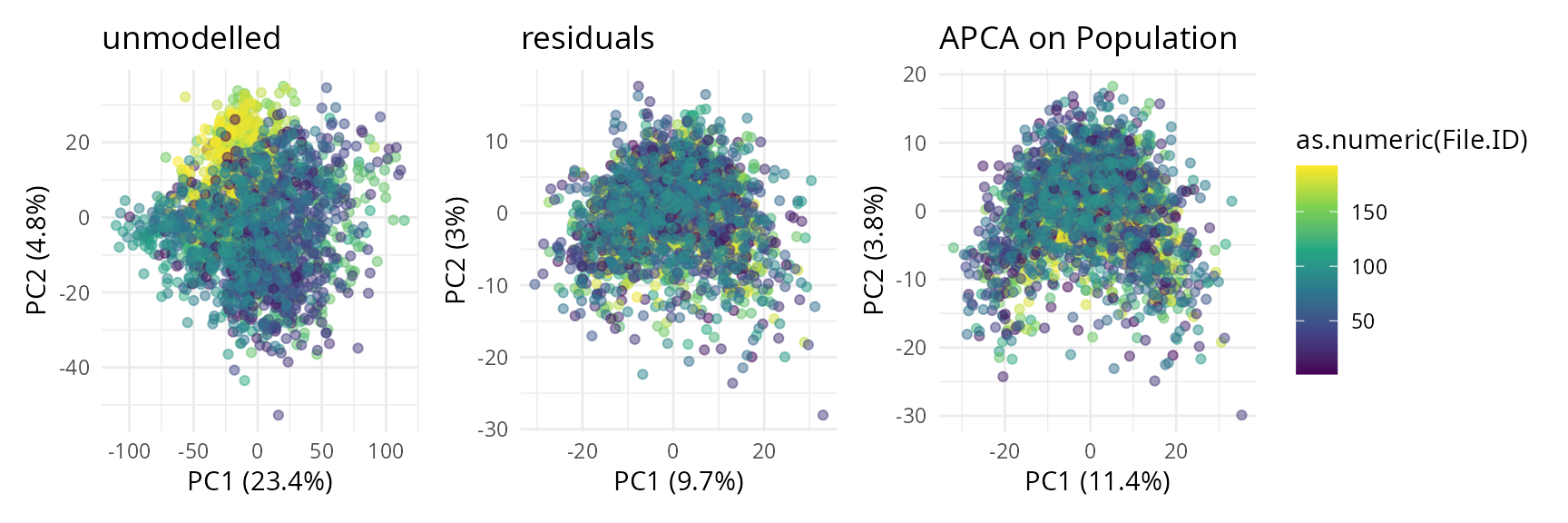
While the data before modelling is driven by batch effects with little biological effects, the APCA shows better separation of the four cell populations, with LSC progressively transitioning to PROG and then BLAST following the PC1 axis. However, strong residuals blurs the differences between populations, indicating that discretising the AML cell population into 4 populations may not be the most relevant approach for these data. We will explore this later during downstream analysis.
We use the same approach to explore the component results in feature space.
caResPeps <- caRes$byFeature
caResPeps <- scpAnnotateResults(
caResPeps, rowData(sce), by = "feature", by2 = "Annotated.Sequence"
)We plot the compenents in peptide-space.
plCApeps <- scpComponentPlot(
caResPeps, pointParams = list(size = 0.8, alpha = 0.4)
)
wrap_plots(plCApeps)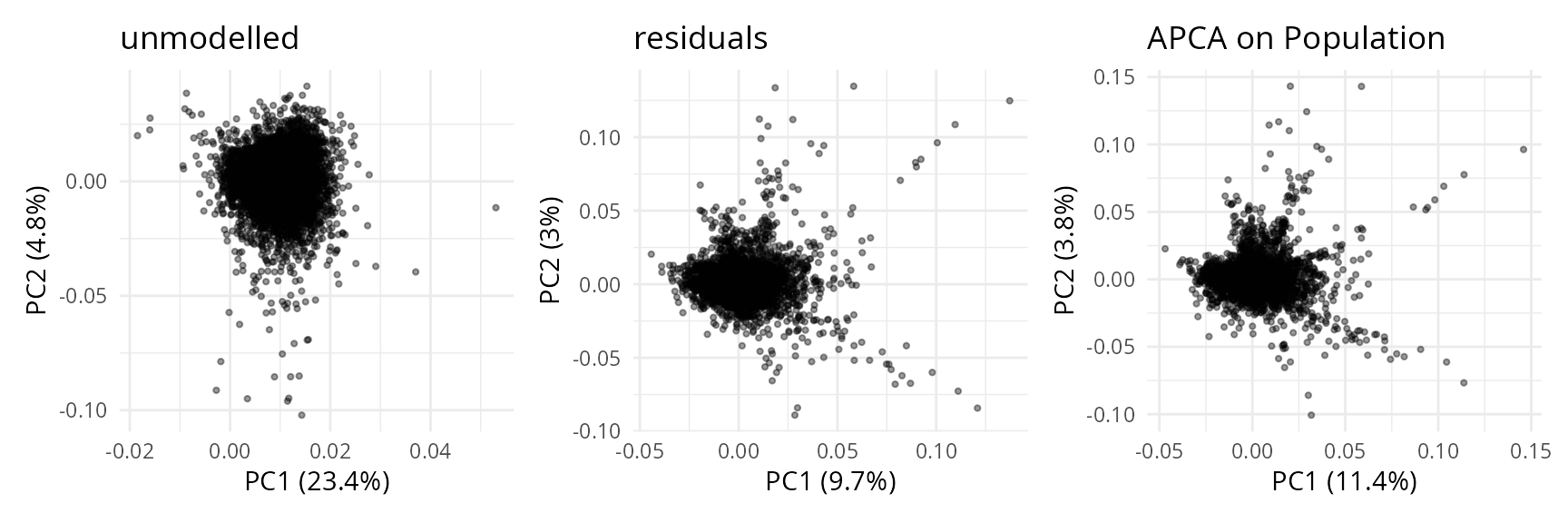
We can also combine the exploration of the components in cell and peptide space. This is possible thanks to biplots.
biplots <- scpComponentBiplot(
caResCells, caResPeps,
pointParams = list(aes(colour = Population)),
labelParams = list(size = 1.5, max.overlaps = 20),
textBy = "Gene", top = 20
)
wrap_plots(biplots, guides = "collect")
## Warning: ggrepel: 14 unlabeled data points (too many overlaps). Consider
## increasing max.overlaps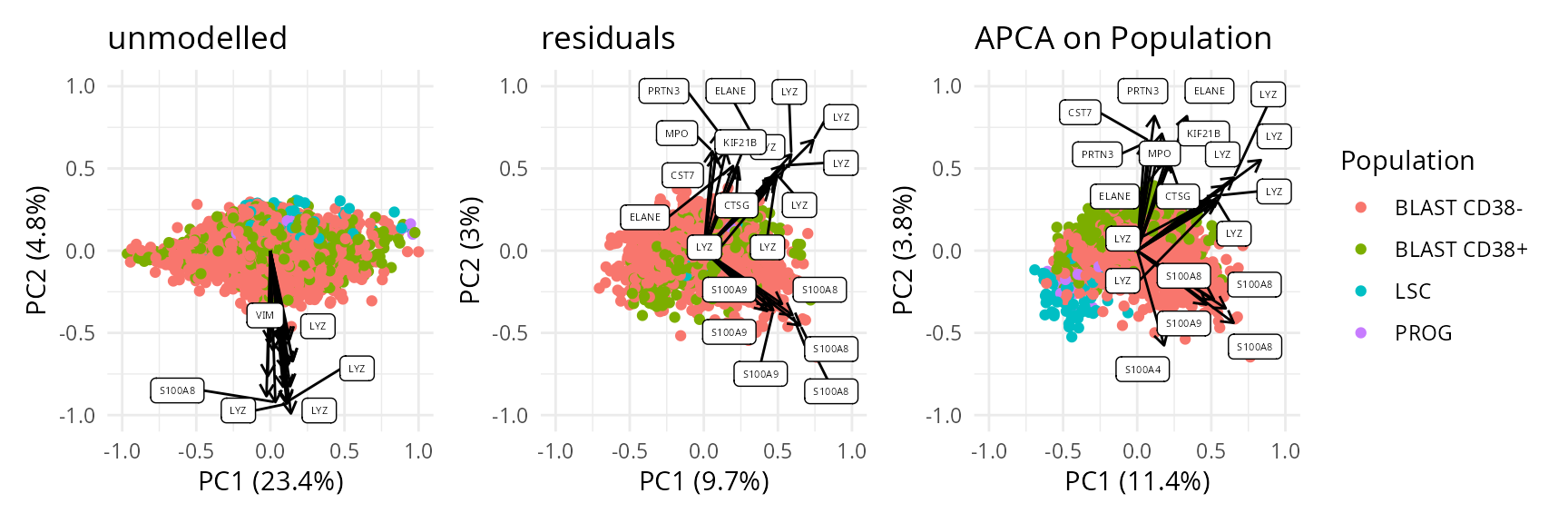
Finally, we offer functionality to aggregate the results at the protein level instead of the peptide level.
caResProts <- scpComponentAggregate(caResPeps, fcol = "Gene")
## Components may no longer be orthogonal after aggregation.
biplots <- scpComponentBiplot(
caResCells, caResProts,
pointParams = list(aes(colour = Population)),
labelParams = list(size = 1.5, max.overlaps = 20),
textBy = "Gene", top = 20
)
wrap_plots(biplots, guides = "collect")
## Warning: ggrepel: 14 unlabeled data points (too many overlaps). Consider
## increasing max.overlaps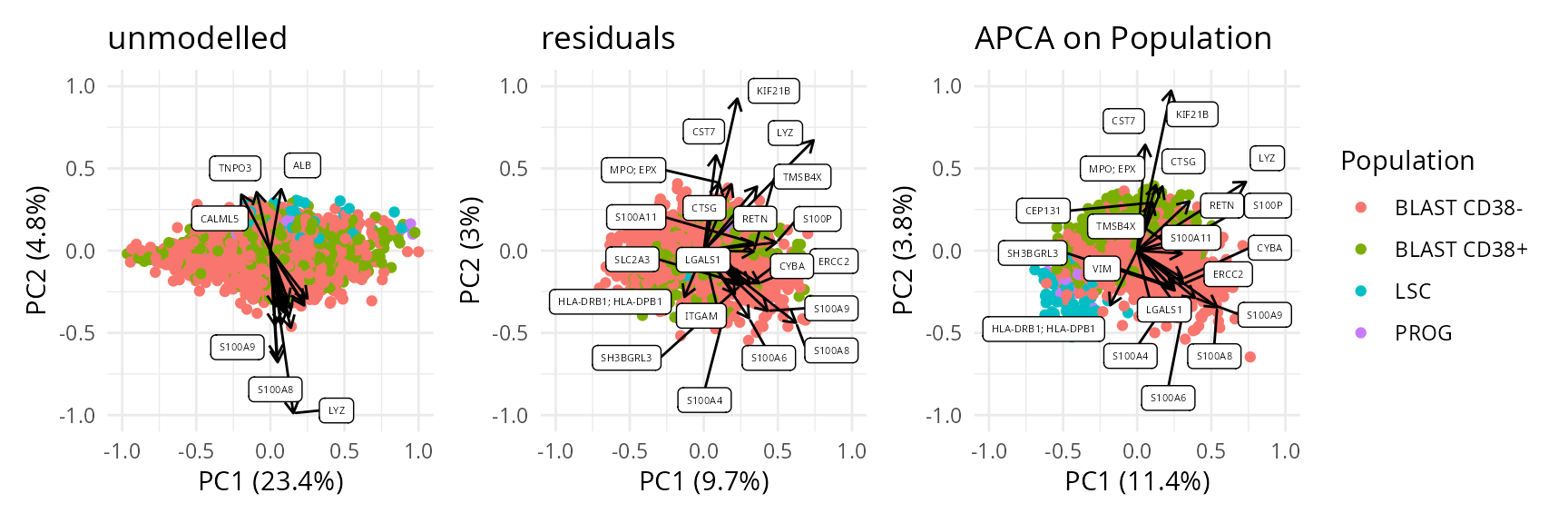
Interactive visualisation with iSEE
You can manually explore the data through an interactive interface
thanks to iSEE:
Downstream analysis
The final step in this analyses in the exploration of the differentiation trajectories of the AML model. To achieve this, we perform an APCA+ with more components to capture more of the variability. The APCA+ results are used to compute diffusion components that are used for diffusion pseudotime ordering.
Difusion pseudo-time analysis
We compute the 20 first APCA+ components on the sample type. We limit the PCA algorithm to 50 iterations to avoid an overly long run and will not compute the PCA for the unmodelled data and the residuals because we won’t use it during the downstream analysis.
apcaPopulation <- scpComponentAnalysis(
sce, ncomp = 20, method = "APCA", effect = "Population",
residuals = FALSE, unmodelled = FALSE, maxiter = 50
)
## [1] "APCA"
## [1] "Population"
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 4.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 6.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 7.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 8.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 9.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 10.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 11.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 12.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 13.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 14.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 15.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 16.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 17.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 18.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 19.
## Warning in nipals(t(mat), ncomp = ncomp, center = center, startcol = startcol, : Stopping after 50 iterations for PC 20.
pca <- apcaPopulation$bySample$APCA_PopulationThe resulting components are stored in the
SingleCellExperiment object.
pca <- as.matrix(pca[, grep("^PC", colnames(pca))])
reducedDim(sce, "APCA") <- pcaWe compute the diffusion map from the PCA results.
dm <- DiffusionMap(pca)
## Warning: 'as(<dsCMatrix>, "dsTMatrix")' is deprecated.
## Use 'as(., "TsparseMatrix")' instead.
## See help("Deprecated") and help("Matrix-deprecated").
reducedDim(sce, "DiffusionMap") <- eigenvectors(dm)Finally, we plot the three first diffusion components.
plot_ly(x = reducedDim(sce, "DiffusionMap")[, 1],
y = reducedDim(sce, "DiffusionMap")[, 2],
z = reducedDim(sce, "DiffusionMap")[, 3],
color = sce$Population,
type = "scatter3d",
mode = "markers")The diffusion map confirms the progressive transition from LSC to BLAST cells. However, we see an alternative differentiation track characterised by low scores for the second diffusion component.
Session information
R Under development (unstable) (2023-07-27 r84768)
Platform: x86_64-pc-linux-gnu
Running under: Ubuntu 23.04
Matrix products: default
BLAS: /usr/lib/x86_64-linux-gnu/blas/libblas.so.3.11.0
LAPACK: /usr/lib/x86_64-linux-gnu/lapack/liblapack.so.3.11.0
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
time zone: Europe/Prague
tzcode source: system (glibc)
attached base packages:
[1] stats4 stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] plotly_4.10.3 scater_1.31.0
[3] scuttle_1.13.0 destiny_3.17.0
[5] EnsDb.Hsapiens.v86_2.99.0 ensembldb_2.27.0
[7] AnnotationFilter_1.27.0 GenomicFeatures_1.55.1
[9] AnnotationDbi_1.65.2 dplyr_1.1.3
[11] patchwork_1.1.3 ggplot2_3.4.4
[13] SingleCellExperiment_1.25.0 scpdata_1.9.2
[15] ExperimentHub_2.11.0 AnnotationHub_3.11.0
[17] BiocFileCache_2.11.1 dbplyr_2.4.0
[19] scp_1.11.3 QFeatures_1.13.0
[21] MultiAssayExperiment_1.29.0 SummarizedExperiment_1.33.0
[23] Biobase_2.63.0 GenomicRanges_1.55.1
[25] GenomeInfoDb_1.39.0 IRanges_2.37.0
[27] S4Vectors_0.41.1 BiocGenerics_0.49.1
[29] MatrixGenerics_1.15.0 matrixStats_1.1.0
[31] BiocStyle_2.31.0
loaded via a namespace (and not attached):
[1] fs_1.6.3 ProtGenerics_1.35.0
[3] bitops_1.0-7 httr_1.4.7
[5] RColorBrewer_1.1-3 tools_4.4.0
[7] utf8_1.2.4 R6_2.5.1
[9] lazyeval_0.2.2 withr_2.5.2
[11] sp_2.1-1 prettyunits_1.2.0
[13] gridExtra_2.3 fdrtool_1.2.17
[15] cli_3.6.1 textshaping_0.3.7
[17] labeling_0.4.3 slam_0.1-50
[19] nipals_0.8 sass_0.4.7
[21] robustbase_0.99-0 proxy_0.4-27
[23] pkgdown_2.0.7 Rsamtools_2.19.2
[25] systemfonts_1.0.5 TTR_0.24.3
[27] rstudioapi_0.15.0 RSQLite_2.3.3
[29] generics_0.1.3 BiocIO_1.13.0
[31] crosstalk_1.2.0 car_3.1-2
[33] Matrix_1.6-1.1 ggbeeswarm_0.7.2
[35] fansi_1.0.5 abind_1.4-5
[37] lifecycle_1.0.4 scatterplot3d_0.3-44
[39] yaml_2.3.7 carData_3.0-5
[41] SparseArray_1.3.0 grid_4.4.0
[43] blob_1.2.4 promises_1.2.1
[45] crayon_1.5.2 lattice_0.22-5
[47] beachmat_2.19.0 KEGGREST_1.43.0
[49] pillar_1.9.0 knitr_1.45
[51] metapod_1.11.0 rjson_0.2.21
[53] boot_1.3-28.1 codetools_0.2-19
[55] glue_1.6.2 pcaMethods_1.95.0
[57] data.table_1.14.8 vcd_1.4-11
[59] vctrs_0.6.4 png_0.1-8
[61] gtable_0.3.4 cachem_1.0.8
[63] xfun_0.41 S4Arrays_1.3.0
[65] mime_0.12 RcppEigen_0.3.3.9.4
[67] interactiveDisplayBase_1.41.0 ellipsis_0.3.2
[69] xts_0.13.1 bit64_4.0.5
[71] progress_1.2.2 filelock_1.0.2
[73] rprojroot_2.0.4 bslib_0.5.1
[75] irlba_2.3.5.1 vipor_0.4.5
[77] colorspace_2.1-0 DBI_1.1.3
[79] nnet_7.3-19 tidyselect_1.2.0
[81] smoother_1.1 bit_4.0.5
[83] compiler_4.4.0 curl_5.1.0
[85] BiocNeighbors_1.21.0 xml2_1.3.5
[87] desc_1.4.2 DelayedArray_0.29.0
[89] bookdown_0.36 rtracklayer_1.63.0
[91] scales_1.2.1 DEoptimR_1.1-3
[93] lmtest_0.9-40 hexbin_1.28.3
[95] rappdirs_0.3.3 stringr_1.5.0
[97] digest_0.6.33 rmarkdown_2.25
[99] XVector_0.43.0 htmltools_0.5.7
[101] pkgconfig_2.0.3 sparseMatrixStats_1.15.0
[103] lpsymphony_1.31.0 highr_0.10
[105] fastmap_1.1.1 rlang_1.1.2
[107] htmlwidgets_1.6.2 ggthemes_4.2.4
[109] shiny_1.7.5.1 DelayedMatrixStats_1.25.0
[111] farver_2.1.1 jquerylib_0.1.4
[113] IHW_1.31.0 zoo_1.8-12
[115] jsonlite_1.8.7 BiocParallel_1.37.0
[117] BiocSingular_1.19.0 RCurl_1.98-1.13
[119] magrittr_2.0.3 GenomeInfoDbData_1.2.11
[121] munsell_0.5.0 Rcpp_1.0.11
[123] viridis_0.6.4 MsCoreUtils_1.15.1
[125] stringi_1.7.12 zlibbioc_1.49.0
[127] MASS_7.3-60.1 parallel_4.4.0
[129] ggrepel_0.9.4 Biostrings_2.71.1
[131] hms_1.1.3 igraph_1.5.1
[133] ranger_0.15.1 RcppHNSW_0.5.0
[135] biomaRt_2.59.0 ScaledMatrix_1.11.0
[137] BiocVersion_3.19.1 XML_3.99-0.15
[139] evaluate_0.23 BiocManager_1.30.22
[141] laeken_0.5.2 httpuv_1.6.12
[143] VIM_6.2.2 tidyr_1.3.0
[145] purrr_1.0.2 knn.covertree_1.0
[147] clue_0.3-65 BiocBaseUtils_1.5.0
[149] rsvd_1.0.5 xtable_1.8-4
[151] restfulr_0.0.15 e1071_1.7-13
[153] RSpectra_0.16-1 later_1.3.1
[155] viridisLite_0.4.2 class_7.3-22
[157] ragg_1.2.6 tibble_3.2.1
[159] memoise_2.0.1 beeswarm_0.4.0
[161] GenomicAlignments_1.39.0 cluster_2.1.4
[163] ggplot.multistats_1.0.0 Citation
citation("scp")
To cite the scp package in publications use:
Vanderaa, Christophe, and Laurent Gatto. 2023. Revisiting the Thorny
Issue of Missing Values in Single-Cell Proteomics. Journal of
Proteome Research 22 (9): 2775–84.
Vanderaa Christophe and Laurent Gatto. The current state of
single-cell proteomics data analysis. Current Protocols 3 (1): e658.;
doi: https://doi.org/10.1002/cpz1.658 (2023).
Vanderaa Christophe and Laurent Gatto. Replication of Single-Cell
Proteomics Data Reveals Important Computational Challenges. Expert
Review of Proteomics, 1–9 (2021).
To see these entries in BibTeX format, use 'print(<citation>,
bibtex=TRUE)', 'toBibtex(.)', or set
'options(citation.bibtex.max=999)'.License
This vignette is distributed under a CC BY-SA license license.